The incidence of synchronous primary lung tumors (SPLT) ranges from 0.2 to 20% and has recently increased due to the widespread use of imaging techniques (such as multidetector computed tomography [CT]) and the increasing implementation of lung cancer screening programs with low-dose radiation thoracic CT.1,2 The simultaneous detection of more than one pulmonary nodule in patients with lung cancer raises the clinical and radiological dilemma of whether these lesions represent intrapulmonary metastases or additional SPLT. Although the classic criteria proposed by Martini and Melamed in 1975 for the diagnosis of SPLT are still in use (based on the histologic features of the tumors, location, presence or absence of carcinoma in situ, vascular invasion, etc.) they do not take into account more modern biomarkers such as driver gene mutations and detailed genetic assessments (like comparative genomic hybridization or Next-Generation sequencing).3,4 Very few cases of three SPLT involving one single lobe have been described,5–7 but to our knowledge there are no cases describing three SPLT in the same lung segment. In this document we describe the case of a patient with three different SPLT in one single pulmonary segment.
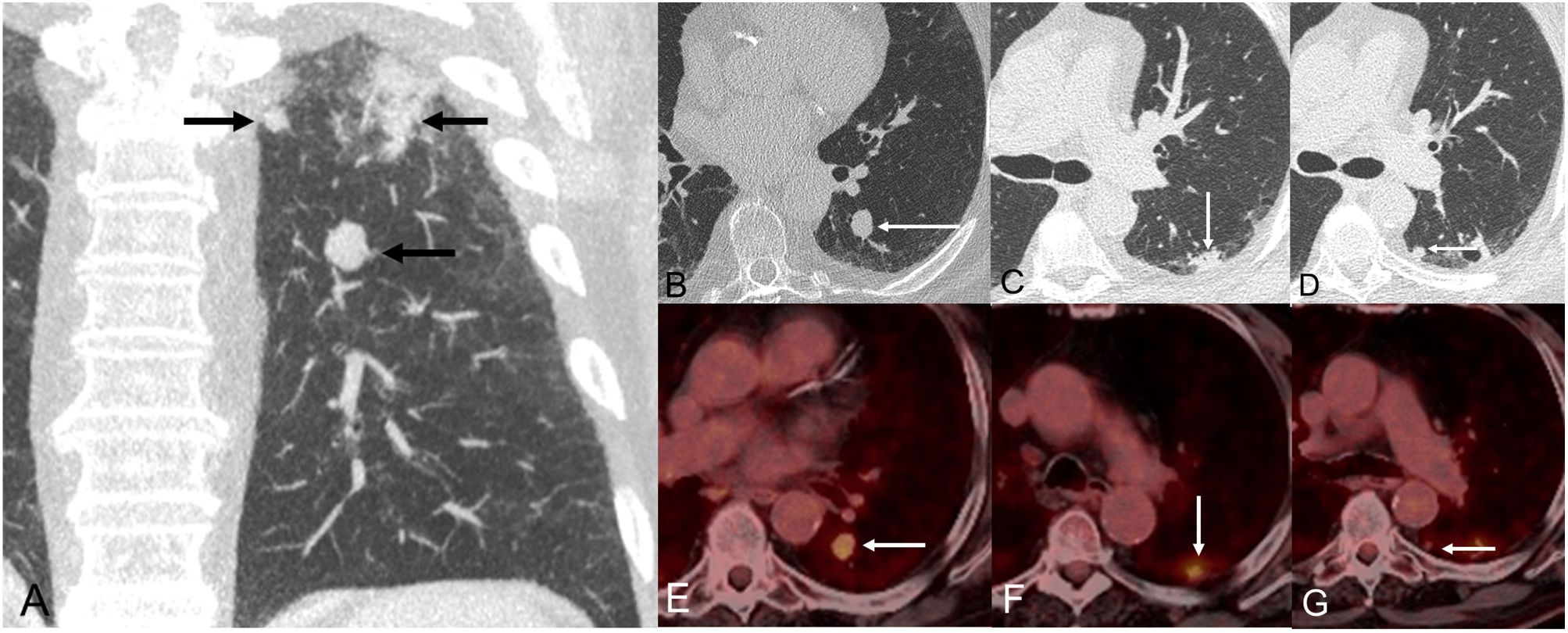
The patient was a 72-year-old male, active smoker, who complained of progressive dyspnea and cough. A chest radiograph showed a nodular opacity in the left lung, so it was decided to perform a thoracic CT, which confirmed the presence of 3 suspicious lesions in the superior segment of the left lower lobe (Fig. 1A): one 16-mm solid nodule in the inferior aspect of the superior segment (lesion 1, Fig. 1B), one 17-mm solid subpleural nodule in the superior and lateral aspect of the superior segment (lesion 2, Fig. 1C), and one 11-mm solid subpleural nodule in the superior and medial aspect of the inferior segment (lesion 3, Fig. 1D). Fluorine-18 fluorodeoxyglucose positron emission tomography/CT (FDG-PET/CT) demonstrated variable standardized uptake values (SUVs) by the three lung nodules: SUV of 4.7 (lesion 1, Fig. 1E), SUV of 4.3 (lesion 2, Fig. 1F), and SUV of 2.2 (lesion 3, Fig. 1G), suggesting a different glycolytic metabolism. There were no signs of mediastinal or distant metastases. Endobronchial ultrasound (EBUS) and bronchial brushing did not show malignant cells. A presumed diagnosis of a T3 (lung cancer associated with ipsilobar nodules) tumor was made, and the patient underwent a video-assisted thoracoscopic left lower lobectomy. Pathologic findings revealed a low-grade malignant neuroendocrine tumor (pT1bN0M0, lesion 1), an invasive acinar-predominant adenocarcinoma (pT1bN0M0, lesion 2), and another invasive acinar-predominant adenocarcinoma (pT1bN0M0). Although the microscopic morphology of lesions 2 and 3 were similar, immunohistochemical differences (Table 1) confirmed that they represented two independent primary lung adenocarcinomas.
(A) Coronal CT image (lung window) shows 3 suspicious lung nodules in the superior segment of the left lower lobe. (B) Axial CT image (lung window) shows one solid nodule in the inferior region of the superior segment (arrow, lesion 1). (C) Axial CT image (lung window) shows one subpleural nodular opacity in the lateral aspect of the superior segment (arrow, lesion 2). (D) Axial CT image (lung window) shows one small solid nodule in the medial aspect of the superior segment (arrow, lesion 3). (E–G) Axial fused PET/CT images corresponding to lesions shown on Figs. B, C, and D, respectively.
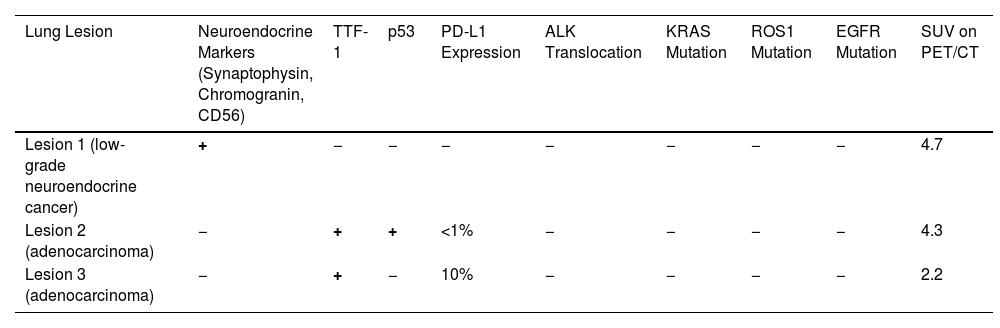
Immunohistochemical Profile and SUV (on PET/CT Imaging) of the Three Synchronous Primary Lung Cancers.
| Lung Lesion | Neuroendocrine Markers (Synaptophysin, Chromogranin, CD56) | TTF-1 | p53 | PD-L1 Expression | ALK Translocation | KRAS Mutation | ROS1 Mutation | EGFR Mutation | SUV on PET/CT |
|---|---|---|---|---|---|---|---|---|---|
| Lesion 1 (low-grade neuroendocrine cancer) | + | − | − | − | − | − | − | − | 4.7 |
| Lesion 2 (adenocarcinoma) | − | + | + | <1% | − | − | − | − | 4.3 |
| Lesion 3 (adenocarcinoma) | − | + | − | 10% | − | − | − | − | 2.2 |
TTF-1: TTF1, thyroid transcription factor-1; p53: p53 tumor suppressor gene; PD-L1: programmed death ligand 1; ALK: anaplastic lymphoma kinase; KRAS: Kirsten rat sarcoma virus; ROS1: ROS Proto-Oncogene 1, receptor tyrosine kinase; EGFR: epidermal growth factor receptor; SUV: standardized uptake value; PET/CT: positron emission tomography/computed tomography.
The simultaneous detection of more than one suspicious lung nodule in the same patient is increasing, due to the widespread use of CTs and the implementation of lung cancer screening programs with low-dose CT.1,2 Distinguishing synchronous primary lung tumors (SPLT) from primary lung cancers with pulmonary metastases has been extensively discussed in the literature, and has implications both on staging and treatment planning, leading to the choice between local therapies for patients with independent primary tumors versus more aggressive systemic therapies for patients with metastatic disease.3,4 Recent improvements in imaging technologies and genomic studies have greatly contributed to the delineation of the clinical, pathologic, and molecular characteristics allowing the discrimination between multiple SPLT and pulmonary metastases.8,9 However, few features are definitive for accurately differentiating intrapulmonary metastasis from SPLT; many commonly used characteristics are suggestive but associated with a substantial rate of misclassification. Therefore, careful review by a multidisciplinary tumor board considering all available information (clinical, imaging, and histopathological findings) is always recommended.10,11 We have only found three reports of three SPLT involving the same lobe.5–7 One of them described two lung cancers in the left upper lobe (one corresponded to a squamous cell cancer and the other one to a combined small cell lung cancer),5 other report described three lesions in the right upper lobe (two invasive lung cancers [one squamous and one adenocarcinoma] and one preneoplastic lung cancer lesion [atypical adenomatous hyperplasia]),6 and another report described three lung cancers in the right lower lobe (one squamous cell cancer, one adenocarcinoma, and one small cell lung cancer).7 Our patient had three primary lung cancers (one typical carcinoid tumor and two invasive adenocarcinomas) in the superior segment of the left lower lobe, but this was only confirmed after thorough immunohistochemical and genetic assessments. The different SUV uptake on FDG-PET/CT by the three lung lesions along with the different expression of p53 and PD-L1 by the 2 lung adenocarcinomas confirmed the independent nature of each lung lesion. Long-term survival after resection for patients with SPLT has been reported to be better than that of patients with higher stages (for reasons other than synchronous tumors).12 Considering this observed survival advantage, surgical resection is presumed to offer the best chance for prolonged survival in these patients; however, controversies related to diagnosis and patient selection for surgical resection still exist. The previously published reports with three SPLT were all surgically treated (lobectomy); in our case, since the presurgical diagnosis suggested a T3 tumor (lung cancer with secondary satellite nodules within the same lobe), a lobectomy was performed. Although a segmentectomy of the superior segment of the left lower lobe could have been attempted, the resection margins for the carcinoid tumor would have been suboptimal.
Synchronous lung cancers in the same (or different) lobe are likely to be underdiagnosed, as it is not always possible to make a histological diagnosis of every lung lesion before treatment initiation (especially if the patient is not a surgical candidate). Multidisciplinary diagnostic evaluation (and comprehensive evaluation of the CT and FDG-PET/CT images) can be very helpful in these cases, because a correct diagnosis will determine the best treatment for the patient and, consequently, a better prognosis.
Conflict of InterestsThe authors state that they have no conflict of interests.