Idiopathic pulmonary fibrosis (IPF) is a chronic fibroproliferative disease characterized by a progressive and irreversible lung scaring with a decline in lung function.1 Transforming Grow Factor Beta (TGF-β) induced myofibroblasts2 are considered as key cells in this pathology, which are responsible of the aberrant fibrosis.1 Recently, mesenchymal stem cells (MSCs) treatment has demonstrated a regenerative effect3 and their extracellular vesicles (EVs) have been established as one of their main therapeutic components.4 Thus, EVs administration as a biological therapy overcomes the drawbacks of cell transplantation.3 Here, we evaluated the potential anti-fibrotic effects of EVs isolated from human umbilical cord MSCs (hUC-MSCs) in a model of lung myofibroblasts.5
First, we established an in vitro primary model of myofibroblasts from lung MSCs (L-MSCs) through TGF-β1 priming. Lung tissues were obtained from donors without history of lung disease (n=3) and with IPF (n=3) under the approval of the ethical committee of Balearic Islands (Code: IB745/06PI). L-MSCs were obtained using previously described methods,6 and their mesenchymality was confirmed by Rohart test.7 Finally, these cells were induced to myofibroblasts with 5ng/ml TGF-β1 for 24h,5 at this time, transforming effects are appreciated by gene expression and functional assays.5
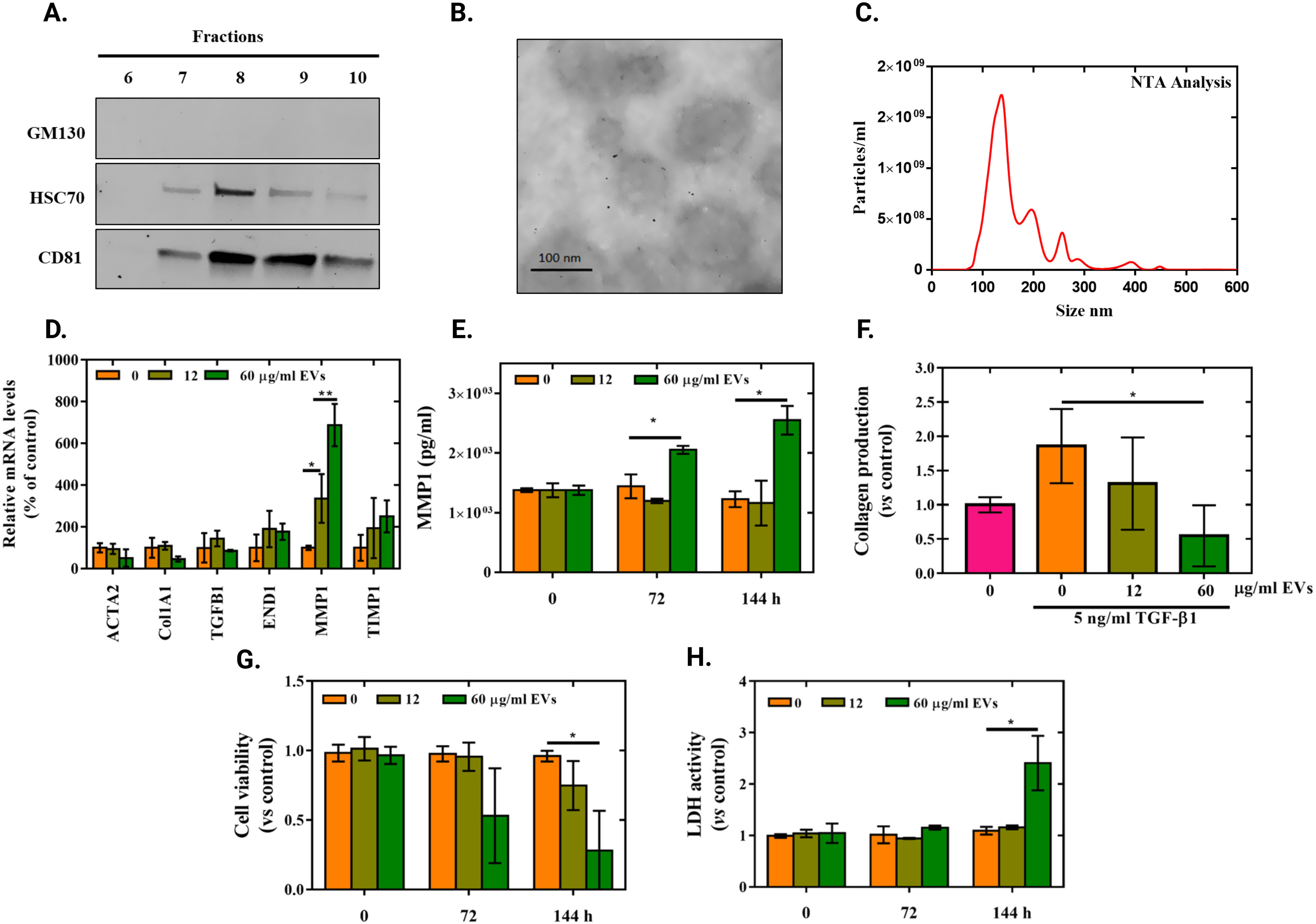
Second, we obtained human umbilical cord MSCs (hUC-MSCs) from the IdISBa Biobank. hUC-MSCs were growth in media supplemented with 10% of platelet lisate (PL) until 80% confluence. Then, cells were washed twice with PBS and cultured with medium without PL (DMEM low glucose supplemented with 2% P/S) for 48h. This conditioned media was employed for EVs isolation by size exclusion chromatography (SEC), followed by ultrafiltration.8 Next, EVs were characterized by biomarker expression, morphology and size by western blot (WB) (Fig. 1A), transmission electronic microscopy (TEM) (Fig. 1B) and nanoparticle tracking analysis (NTA) (Fig. 1C) respectively, according to the previously described methodology.8
EVs characterization and effect in myofibroblast from L-MSCs. hUC-MSCs culture supernatant was concentrated to 5ml by tangential flow filtration and immediately injected into a SEC column and fractioned in 5ml fractions. (A) Presence of specific EVs markers (HSC70 (clone B-6, Santa Cruz, USA) and CD81 (clone B-11, Santa Cruz, USA)) and lack of GM130 (clone N3C2, Santa Cruz, USA) evaluated by WB in the collected fractions. Higher expression of EVs marker can be observed at fractions 8 and 9, corresponding with higher UV absorbance in the chromatogram. (C) Representative TEM image of isolated EVs. (D) NTA analysis of pulled EV enriched fractions (7–10) shows particles with 163nm mean size. (D) Gene expression of fibrosis related genes. Data represent fold changes of target genes normalized to beta-actin and GAPDH (reference genes) expressed relative to control that was set to 100% (n=3). (E) MMP1 protein levels released to cell culture media at 0, 72 and 144h after treatment (n=3). (F) Quantification of the Sirius Red stained collagen in TGF-β1 induced myofibroblasts. Data represents fold changes relative to non TGF-β1 primed L-MSCs (n=3). (G) Cell viability measured after 0, 72 and 144h of EVs treatment in TGF-β1 induced myofibroblasts. Data represent fold changes relative to non-treated TGF-β1 primed cells at each time point (n=3). (H) LDH activity in cell culture media after 0, 72 and 144h of EVs treatment in TGF-β1 primed cells. Data represent fold changes relative to non-treated TGF-β1 primed cells at each time point (n=3).
Bar graphs represent the mean±S.D. Multiple t student and ANOVA tests were performed to assess differences: *p<0.05; **p<0.005.
For EVs treatment, we set two EVs’ concentrations (12 and 60μg/ml) based on total protein measurement at 280nm using a NanoDrop; these concentrations corresponded to 1.4×1010 and 7.3×1010particles/ml by NTA respectively. Then, we treated myofibroblasts from L-MSCs during 24h to determine expression of fibrotic related genes. This analysis showed not significant differences in ACTA2, Col1A1, TGF-β1, END1 and TIMP1 (Fig. 1D). However, a significant dose-dependent increase on MMP1 mRNA expression was observed (Fig. 1D). It was confirmed by increased MMP1 protein release (measured by ELISA) at 72 and 144h after treatment for the higher dose (60μg/ml) of EVs (Fig. 1E). In this line, a significant reduction of collagen deposits (stained with Sirius Red and quantified at 540nm9) was observed in cells treated with the higher EVs concentration for 144h (Fig. 1F). It is important to remark that, 72 and 144h were set as the time needed to observe MMP1release and its effects in collagen deposits, as previously described in related works.10 Next, we performed a Presto Blue assay according to manufacturer's instructions (Life Technologies, CA, USA) to evaluate cell viability and a LDH activity according to manufacturer's protocol (Roche Diagnostics, Manheim, Germany) to evaluate cytotoxicity as other possible causes of collagen reduction. The obtained results indicate that the collagen reduction induced by the high dose EVs treatment was accompanied by a significant decrease on cell viability and increased cell cytotoxicity at 72 and 144h (Fig. 1G and H). Though, this effect was not observed if L-MSCs were not previously primed with TGF-β1.
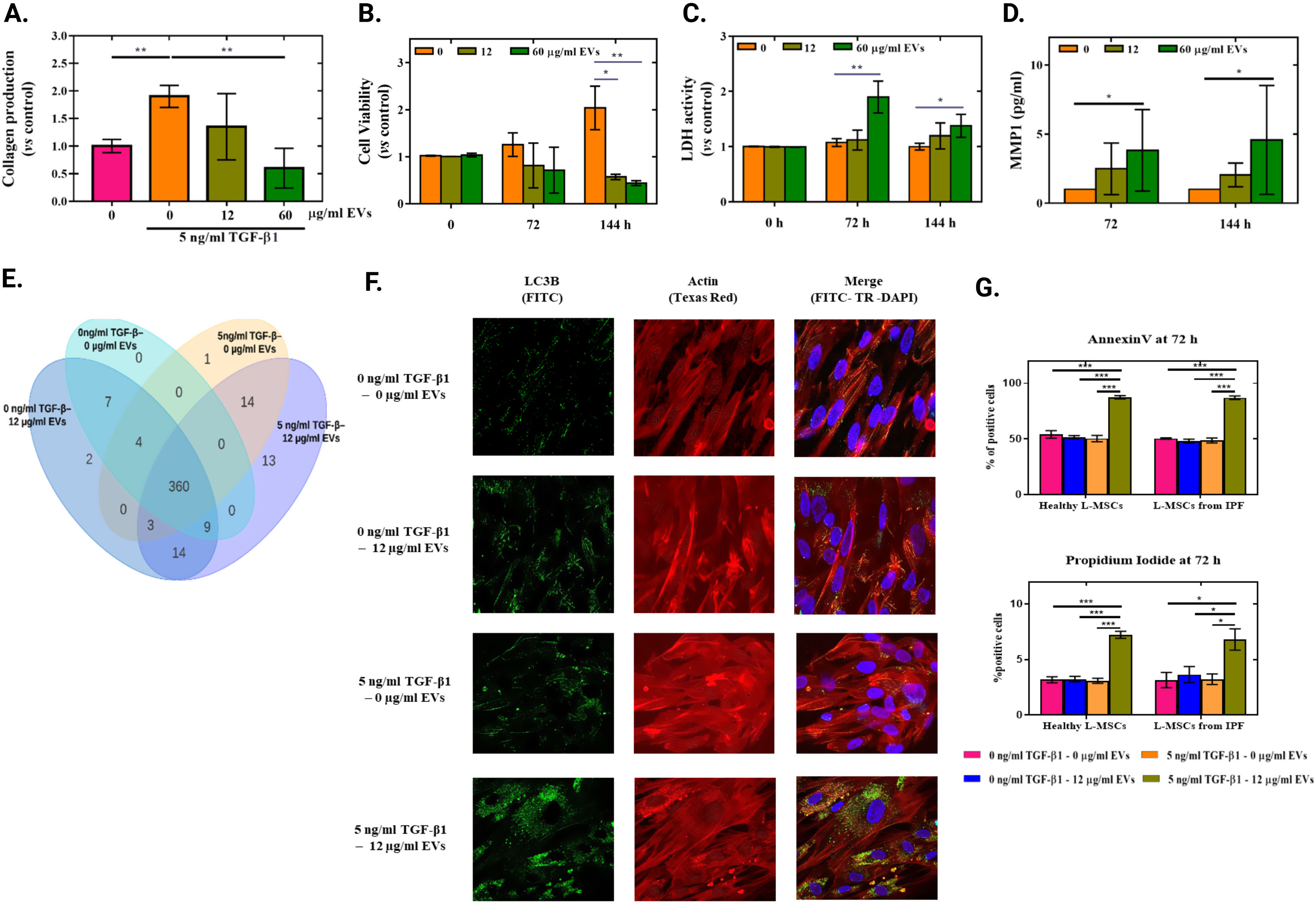
Then, to evaluate the translational importance of our findings, we obtained TGF-β1 induced myofibroblasts from MSCs of IPF lung tissues (IPF-MSCs). We treated them with EVs derived from hUC-MSCs, as previously described. As seen in Fig. 2, treatment with EVs induced the same effects on cells derived from IPF patients. A significant reduction on collagen deposits and on cell viability was observed when cells were primed with TGF-β1 (Fig. 2A and B) as well as a significant increase on released LDH and MMP1 to cell culture media when cells were treated with the highest EVs concentration (60μg/ml) (Fig. 2C and D). It is important to highlight that, in any case, EVs do not produce significant differences, if IPF-MSCs have not previously been transformed by TGF-β1.
Effects of EV in myofibroblasts from IPF-MSCs. Quantification of Sirius Red stained collagen in TGF-β1 primed cells. Data represents fold changes relative to non TGF-β1 primed cells (n=3). (B) Cell viability measured after 0, 72 and 144h of EVs treatment in TGF-β1 primed cells. Data represents fold changes relative to non EVs treated cells at each time point (n=3). (C) LDH activity released to cell culture media after 0, 72 and 144h of EVs treatment in TGF-β1 primed cells. Data represent fold changes relative to non EVs treated cells at each time point (n=3). (D) MMP1 protein levels released to cell culture media at 72 and 144h after EVs treatment in TGF-β1 primed cells (n=3). (E) Proteomic analysis of 483 total and phosphorylated proteins were analysed by reverse phase protein array (RPPA) of IPF-MSCs from the same patient under 4 conditions: non treated L-MSCs, non TGF-β1 primed L-MSCs+12μg/ml EVs, TGF-β primed L-MSCs without EVs treatment and TGF-β1 primed L-MSCs+12μg/ml EVs, Venn diagram showing differentially expressed (over 0.3 Log2 median-centered) proteins between groups. (F) Representative confocal images of cells stained for LC3-B in green (FITC), actin in red (Phalloidin-Texas red) and nuclei in blue (DAPI). (G) Graphical representation of annexin V and propidium iodide positive cells at 72h of EVs treatment (n=3).
Bar graphs represent the mean±S.D. Multiple t student and ANOVA tests were performed to assess differences: *p<0.05; **p<0.005; ***p<0.0005.
Next, just to confirm differential proteomic signature between treatments, we performed an experiment that included 4 groups: non treated IPF-MSCs, non TGF-β1 primed IPF-MSCs+12μg/ml EVs, TGF-β primed IPF-MSCs without EVs treatment and TGF-β1 primed L-MSCs+12μg/ml EVs. Here, we set an overnight treatment time to allow the visualization of protein degradation and production de novo.11 After treatment, the cells were lysed and analysed by reverse phase protein array (RPPA) at the proteomic facilities of MDAnderson cancer centre (Dallas, USA) according with their protocols.12 This analysis of 483 proteins demonstrated that several proteins are differentially expressed in each kind of treatment and 13 proteins (metabolism related proteins) differentially expressed in TGF-β1 primed IPF-MSCs when treated with EVs, as shown in the VENN diagram (Fig. 2E); confirming in this manner a differential signature. Additionally, because autophagy is a secondary consequence of metabolic alteration,13 we evaluated autophagy after 72h of EVs treatment by identification of microtubule-associated proteins 1A/1B light chain 3B (LC3B (NB100-2220 NOVUS, Colorado, USA)) using immune fluorescence (IF) to identify auto-phagosomes. We observed an increased number of autophagosomes in TGF-β1 primed IPF-MSCs when treated with EVs (Fig. 2F). Finally, we wanted to know if EVs induce apoptosis in TGF-β1 primed cells. Thus, at 72h of EVs treatment we performed annexin V and propidium iodide analysis by flow cytometry, using the apoptosis AnnexinV kit (AbCam Cambridge, UK), following the supplier recommendations. In fact, we observed a significant increase of annexin V positive cells on TGF-β1 primed MSCs when treated with EVs compared with the other groups in both, L-MSCs (87.47% vs 54.07%) and IPF-MSCs (87.07% vs 50.53%). Showing the same pattern for PI positive cells (7.23% vs 3.23% in L-MSCs and 6.81% vs 3.64% in IPF-MSCs) (Fig. 2G). These results are in agreement with the reduced cell viability observed in TGF-β1 primed cells when treated with EVs. Interestingly, a major inhibition in the model obtained from IPF-MSCs was observed. Possibly because IPF-MSCs are aged cells with signs of senescence14 and decreased plasticity to adapt their behaviour to resist under challenging conditions.15
Summarizing, in this work we have showed that EVs from hUC-MSCs generate a specific modulation of myofibroblasts originated by priming L-MSCs and IPF-MSCs with TGF-β1 obtained. This myofibroblasts are part of the fibroblastic loci and responsible of the excessive deposition of disorganised collagen and extracellular matrix, all in all resulting in the distortion of the normal lung architecture.1 Here, we have showed that EVs treatment produce an increase of collagenase MMP1, a reduction of collagen deposits, and a reduction of myofibroblasts cell viability with increased autophagy and apoptosis. Although for clinical translation there is a need to improve EVs production process and to assess under in vivo settings its kinetics, biodistribution, efficacy and tolerability; our findings increase the scientific evidence for the use of EVs as treatment in fibrosis diseases, representing a hope for IPF treatment.
Conflict of InterestsM. Molina-Molina has received grants and funding from: Roche, Boehringer, Esteve-Teijin, Pfizer, Chiesi, Astra-Zeneca, Galapagos. The other authors have no conflicts of interest.
We specially thank to the IPF patients who selflessly gave their tissue samples for this study. This research was funded by the Instituto de Salud Carlos III (MS16/00124; CP16/00124; PI19/01578).