Patients with severe Chronic Obstructive Pulmonary Disease (COPD) often experience breathlessness and exercise limitations due to expiratory flow limitation. Pulmonary rehabilitation programmes, including exercise with non-invasive ventilation (NIV) or high-flow nasal therapy (HFT), aims to improve quality of life and exercise tolerance. This study investigates the relationship between thoracoabdominal asynchrony (TAA) during supported (NIV and HFT) and unsupported (conventional oxygen therapy – COT) exercise and clinical and functional parameters in severe COPD patients awaiting lung transplantation.
MethodsThis experimental, longitudinal, prospective, controlled study included 20 severe COPD patients on the lung transplant waiting list. Patients underwent three constant load exercise tests under COT, NIV, and HFT conditions. TAA was measured using respiratory inductance plethysmography, and neurorrespiratory drive (NRD) was assessed via parasternal electromyography.
ResultsPatients exhibited distinct TAA patterns during exercise. Clockwise rotation (thorax ahead) was associated with worse baseline lung function, higher peak exercise dyspnoea and higher peak exercise NRD compared to counterclockwise rotation (abdomen ahead). No significant differences in TAA were observed between the three exercise conditions (COT, NIV, HFT). However, patients with clockwise TAA were more likely to have reduction in breathlessness with NIV compared to COT than those with counterclockwise rotation.
ConclusionsTAA patterns during exercise in severe COPD patients can indicate the severity of lung function impairment and predict severity of exercise induced dyspnoea. Analysis of TAA may predict response to respiratory support modalities and therefore monitoring TAA and NRD should be further studied to allow better tailoring of respiratory support during rehabilitation.
Patients with severe Chronic Obstructive Pulmonary Disease (COPD) experience breathlessness and exercise limitation due to expiratory flow limitation.1 Pulmonary rehabilitation programmes are key components of care for stable COPD, improving quality of life, preventing exacerbations, and being cost-effective. Outpatient programmes include regular training sessions (3–5 times per week) at 60–80% of peak exercise capacity.2
Exercise rehabilitation can be supported by conventional oxygen therapy (COT), non-invasive ventilation (NIV), or high-flow nasal therapy (HFT). NIV improves acute exercise capacity by reducing the work of breathing but requires expertise, and its long-term impact is uncertain. Current data indicates NIV is optimal for augmenting exercise performance in stable COPD.2,3
NIV monitoring during exercise includes techniques such as oxygen saturation, transcutaneous CO2,4 pulmonary function testing, respiratory muscle electromyography (EMG)5 or thoracoabdominal asynchrony (TAA) evaluation using optoelectronic or respiratory inductance plethysmography (RIP).6 TAA, caused by impaired airway obstruction, increases work of breathing and dyspnoea and reduces exercise capacity. A new method proposes the use of Global Phase Delay (GPD) for improved accuracy.7
This study aims to determine the relationship between TAA (using GPD) during supported (NIV and HFT) and unsupported (COT) exercise and clinical and functional parameters in severe COPD patients who are candidates for lung transplantation.
MethodsThe study design was an experimental, longitudinal, prospective, non randomized controlled study with three sequential protocols (exercise testing supported by: COT, NIV and HFT). The before/after comparison of the same individuals was conducted. Exercise testing was performed in a single centre (Hospital Universitario 12 de Octubre), and the signal processing and calculations were made by the team at Hospital Universitari Parc Tauli (Sabadell) and Hospital 12 de Octubre.
PopulationInclusion Criteria- •
The study included patients with severe COPD (with an obstructive pattern and air trapping, according to GOLD criteria, and a residual volume>120% of predicted), admitted on the lung transplant waiting list. These patients were evaluated by the Lung Transplant Unit between September 2019 and November 2022. Comorbidities as confounding factors were excluded as a thorough evaluation was made to be included in lung transplant list.
- •
Patients had expiratory flow limitation by the analysis of flow/volume curves during exercise. This fact was assessed subjectively by 2 experienced operators while performing initial adaptation to NIV during exercise.
- •
Patients were already adapted and compliant with home NIV as a bridge to transplantation.
- •
The patient must not have any uncontrollable comorbidities that limit their ability to exercise. These include uncontrolled ischaemic heart disease, severe pulmonary hypertension and neuromuscular pathology.
- •
Refusal of treatment with NIV or enrolment in the study.
- •
Inability to perform the proposed exercise under baseline conditions and with ventilation.
- •
Lung transplantation during the study resulted in incomplete exercise sessions.
In a first visit, a “ramp test” with incremental loads up to maximal tolerated was performed. Then, a 75% load of that maximal tolerated one was selected to perform the tests. Patients were already included in the exercise protocol for lung transplantation programme, so they all have previously performed at least three sessions of exercise in cycloergometer under COT. Three constant load exercise tests were performed over three consecutive days. The first day was performed under COT, and at the end of the exercise testing, a 5min trial with NIV, cycling with no strict protocol, was performed to titrate pressures. The NIV and the HFT trials were then randomly performed in subsequent days. All of the patients underwent exercise with an Astral 150® ventilator (Resmed, Ca, USA®) under ST mode, as it has been tested previously capable of providing enough pressurization support and capable of different programs.8 Expiratory pressure was titrated to avoid ineffective efforts and trigger delay, and Inspiratory pressure was titrated in order to diminish at least 20% of neurorrespiratory drive (NRD) as measured by parasternal EMG. To avoid cross contamination with pectoral muscle activity (due to grip over the handlebar9), a recumbent cycloergometer (SanaComfort 1000, Ergosana GmbH, Blitz, Germany) was used for the all three exercise tests. The subjects were instructed to cycle with their arms relaxed along their trunk and avoid grasping the handlebars or support bars.
The exercise tests comprised 10min of exercise and 5min of recovery. They were conducted under the following conditions:
- •
COT: Patients will start under their home COT flow and then titrated to maintain an SpO2>92% through all the exercise.
- •
NIV: titrated as described above, with supplemental oxygen if needed, to maintain SpO2>92%.
- •
HFT (Airvo 2®, Fisher & Paykel, Auckland, NZ): A flow of 40l per minute was selected. Supplemental O2 flow was initially set – before starting exercise – to maintain an estimated FiO2 similar to that obtained to achieve a SpO2>92% with COT.10
Before the exercise, we performed three inhalation manoeuvres to measure maximum inspiratory pressure with simultaneous EMG recording for calibration (see below). No other pulmonary function was measured during or after the exercise.
Signal RecordingDuring the test, patients were connected to a recording system equipped with the following sensors:
- -
Parasternal surface EMG is performed with electrodes placed on both sides of the second parasternal space, as previously described.5
- -
Combined transcutaneous CO2 and pulse oximetry oxygen saturation sensor (Sentec TCM®, Therwill, Switzerland).
- -
Chest and abdominal RIP belts (Braebon QZ-RIP, Braebon Medical Corp, Ontario, Canada).
All sensors were connected to a digital-to-analogue converter (PowerLab SP, ADInstruments, Australia). The sampling frequency was set to 2000Hz.
Data Processing- -
RIP signal was filtered with a 2Hz low-pass filter to eliminate the artefact caused by the cycling activity over the abdominal belt. The filtered signals were exported to Matlab® to compute GPD7 (See online supplement for details).
- -
Parasternal EMG signal: An 80Hz high-pass filter was applied. The root mean square (RMS) was calculated as previously described.11 We avoided non-respiratory tonic artefacts in the EMG by estimating the onset of inspiration from the RIP signal. Finally, we related both the peak and the area under the peak of the RMS signal to the mean baseline EMG activity, expressing the result as a percentage of it.12
The following variables were collected. Anthropometric data and pulmonary function tests were conducted (maximum, three months prior to the exercise test). These included forced spirometry, whole-body plethysmography and arterial blood gases. The BODE index and prescribed ventilator parameters were also recorded.
The following variables were recorded during the exercise test at baseline, 1, 4, 7 and 10min of exercise and after 3 and 5min of recovery:
- -
Dyspnoea, measured by the Borg scale.
- -
Global Phase Delay (mean value of the last 5 cycles of each of the previously stated stages).
- -
EMG variables: A maximal inspiratory manoeuvre was performed before each day of testing. The parasternal EMG signal was defined as a fraction of the 100% maximal EMG signal obtained during the maximal inspiratory pressure manoeuvre.
- -
Clockwise rotation: advance of the thoracic belt and assign a positive sign to GPD.
- -
Anticlockwise rotation: abdominal belt was advanced over the thoracic belt, and a negative sign was assigned to GPD.
- -
Thoracic paradox: anticlockwise rotation with a GPD between −90 and −180° in any step of the exercise.
- -
Abdominal paradox: clockwise rotation with a GPD between +90 and +180° in any step of the exercise.
We calculated the NRD as the product of the respiratory rate and both the peak and area under the peak RMS as described by Jolley et al.13
Statistical AnalysisThe Kolmogorov–Smirnov test analyzed the normality of quantitative variables. For normal distributions, the mean and standard deviation were provided; otherwise, the median and interquartile range were given. The Student t test for non-paired measures compared TAA groups. A mixed ANOVA for repeated measures assessed performance across exercise tests. The F score, mean differences, and observed power (β−1) were provided. Significance was set at p<0.05. Statistical analyses were performed using SPSS version 25.
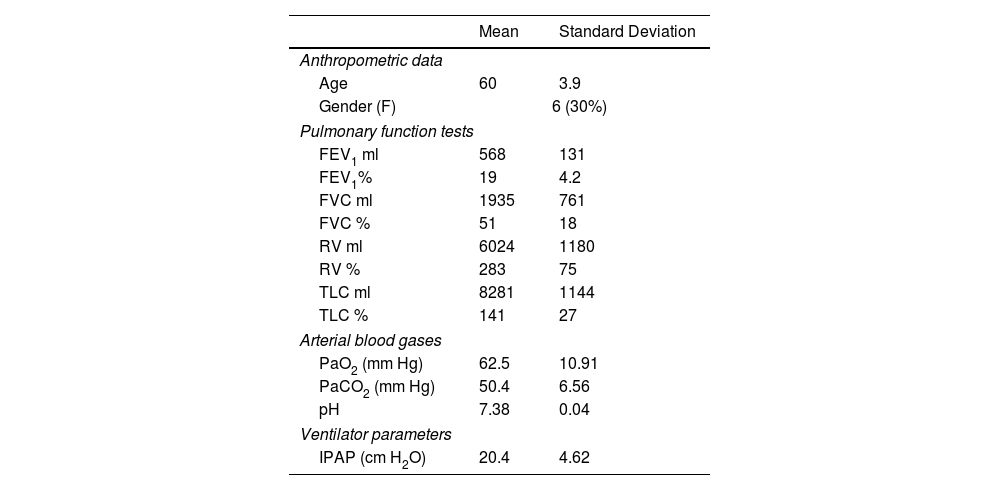
ResultsThrough the study period, 47 patients with COPD were included in the lung transplant programme of whom 23 were on home NIV and suitable for inclusion. One patient underwent lung transplantation before the trial could be conducted and 2 did not have reliable EMG signals despite several attempts. The study population therefore included 20 patients with severe COPD (6 women). The anthropometric data, pulmonary function tests and ventilator parameters for the entire cohort are shown in Table 1.
Anthropometric and Lung Function Data for the Entire Cohort.
| Mean | Standard Deviation | |
|---|---|---|
| Anthropometric data | ||
| Age | 60 | 3.9 |
| Gender (F) | 6 (30%) | |
| Pulmonary function tests | ||
| FEV1 ml | 568 | 131 |
| FEV1% | 19 | 4.2 |
| FVC ml | 1935 | 761 |
| FVC % | 51 | 18 |
| RV ml | 6024 | 1180 |
| RV % | 283 | 75 |
| TLC ml | 8281 | 1144 |
| TLC % | 141 | 27 |
| Arterial blood gases | ||
| PaO2 (mm Hg) | 62.5 | 10.91 |
| PaCO2 (mm Hg) | 50.4 | 6.56 |
| pH | 7.38 | 0.04 |
| Ventilator parameters | ||
| IPAP (cm H2O) | 20.4 | 4.62 |
The study population had a median maximum workload of 25W (range 7–60W) as determined in the baseline ramp test. All patients had a BODE index of 7 or more (median 9, range 7–12). Extensive cardiac examinations, including cardiac ultrasound and coronariography, excluded significant cardiovascular disease. Mean MIP was −65cm H2O, suggesting diaphragm weakness was not the primary cause of exercise limitation. All patients exhibited air trapping, with a mean FEV1 of 19±4% and a mean residual volume of 283±75%. All of the patients had stable condition hypercapnia. Thus, dynamic hyperinflation was suspected as the main driver of exercise limitation.
Baseline mean GPD: 14 patients had counterclockwise rotation (abdomen ahead) with a mean GPD of −14.24±15.56 and 6 had clockwise rotation (thorax ahead) with a mean GPD of 23.47±22.69. Patients with clockwise rotation had a lower percent predicted FEV1 and FVC than patients with counterclockwise rotation, with no significant differences in the other lung function parameters (FEV1 15.5%±3.44% vs. 20.54±3.85%, p<0.05 and FVC 37.5±13.86 vs. 54.50±18.14, p<0.05).
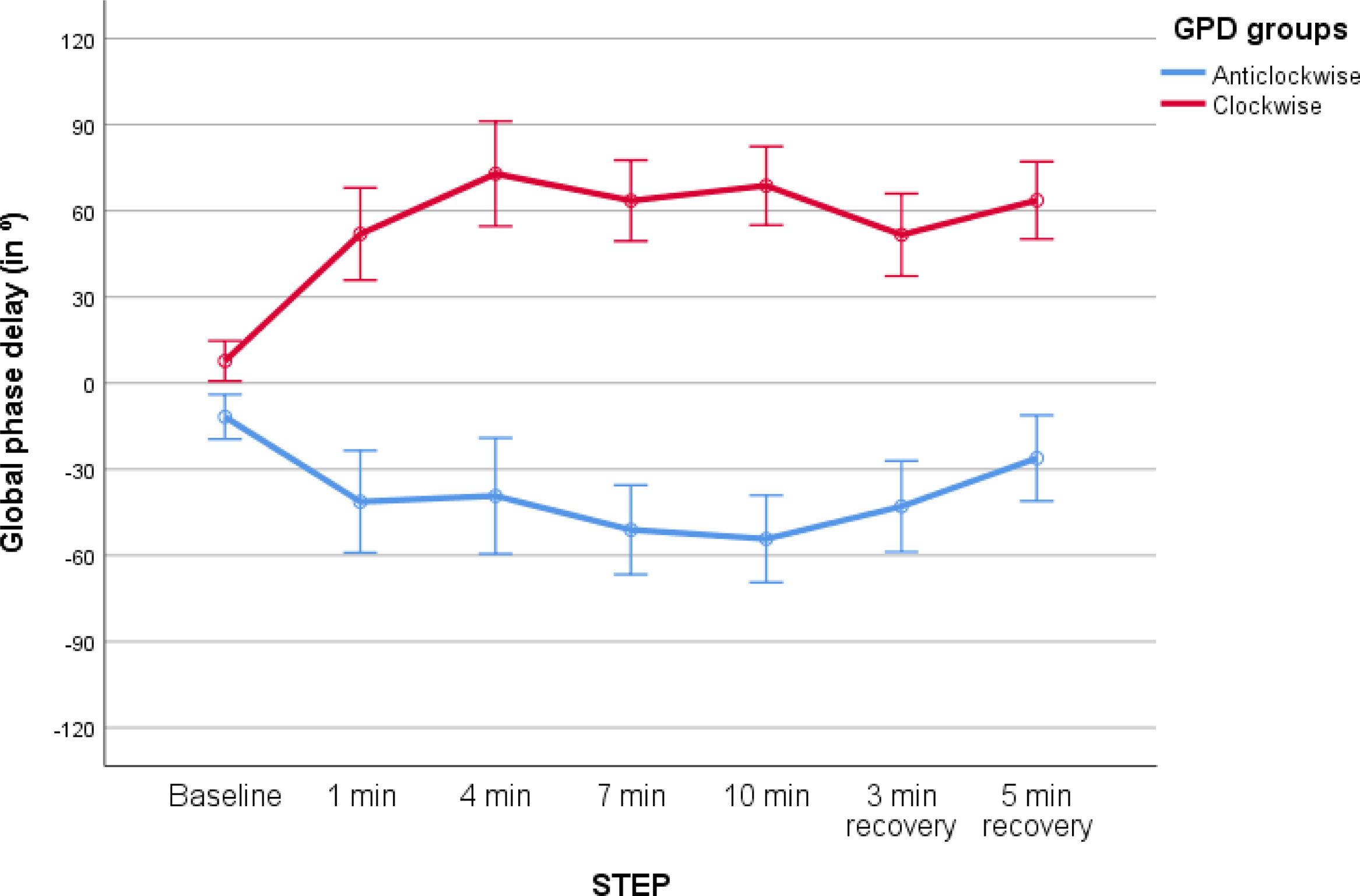
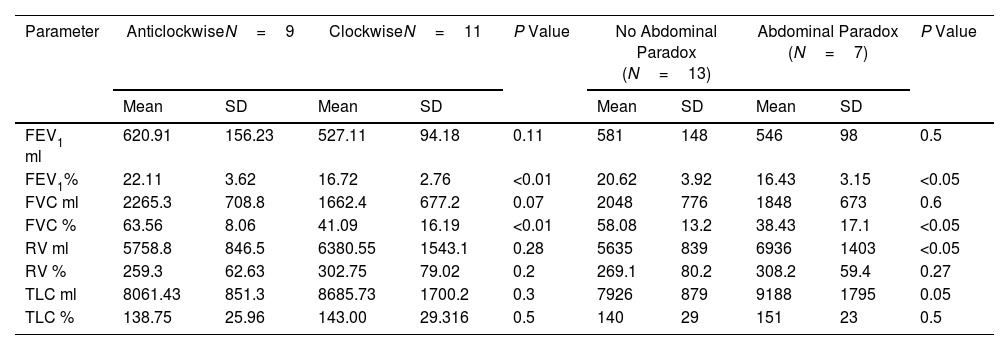
Exercise Tests With COTDuring exercise on COT 9 patients change the pattern of breathing to counterclockwise rotation (2 with thoracic paradox at some point during exercise), while 11 had clockwise rotation (7 with abdominal paradox at some point during exercise), see Fig. 1. Significant differences were observed between groups (F=23.47, p<0.01, mean difference between groups 88.4°, 95% CI 50.07–123.76, (β−1)=0.99 mixed ANOVA for repeated measures). Table 2 shows the differences in lung function between patients with clockwise and counterclockwise rotation, and in patients with and without abdominal paradox.
Global Phase Delay (GPD) performance during exercise with conventional oxygen therapy in the study population. Two distinct groups were identified: (1) a group with predominant anticlockwise rotation (abdomen ahead, blue line), and (2) a group with clockwise rotation (thorax ahead, red line), showing higher absolute deviations compared to the former (p<0.01, ANOVA for repeated measures).
Thoracoabdominal Synchrony During Exercise With Conventional Oxygen Therapy and Group Correlation Based on TAA and Pulmonary Function Tests.
| Parameter | AnticlockwiseN=9 | ClockwiseN=11 | P Value | No Abdominal Paradox (N=13) | Abdominal Paradox (N=7) | P Value | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |||
| FEV1 ml | 620.91 | 156.23 | 527.11 | 94.18 | 0.11 | 581 | 148 | 546 | 98 | 0.5 |
| FEV1% | 22.11 | 3.62 | 16.72 | 2.76 | <0.01 | 20.62 | 3.92 | 16.43 | 3.15 | <0.05 |
| FVC ml | 2265.3 | 708.8 | 1662.4 | 677.2 | 0.07 | 2048 | 776 | 1848 | 673 | 0.6 |
| FVC % | 63.56 | 8.06 | 41.09 | 16.19 | <0.01 | 58.08 | 13.2 | 38.43 | 17.1 | <0.05 |
| RV ml | 5758.8 | 846.5 | 6380.55 | 1543.1 | 0.28 | 5635 | 839 | 6936 | 1403 | <0.05 |
| RV % | 259.3 | 62.63 | 302.75 | 79.02 | 0.2 | 269.1 | 80.2 | 308.2 | 59.4 | 0.27 |
| TLC ml | 8061.43 | 851.3 | 8685.73 | 1700.2 | 0.3 | 7926 | 879 | 9188 | 1795 | 0.05 |
| TLC % | 138.75 | 25.96 | 143.00 | 29.316 | 0.5 | 140 | 29 | 151 | 23 | 0.5 |
Two comparisons are presented: (1) clockwise (thorax ahead) vs anticlockwise (abdomen ahead) in columns 2 and 3, and (2) abdominal paradox (indicative of extreme diaphragm weakness/fatigue) vs no abdominal paradox in columns 5 and 6.
Abbreviations: FEV1: forced expiratory volume in the first second, FVC: forced vital capacity, RV: residual volume; TLC: total lung capacity; SD: standard deviation.
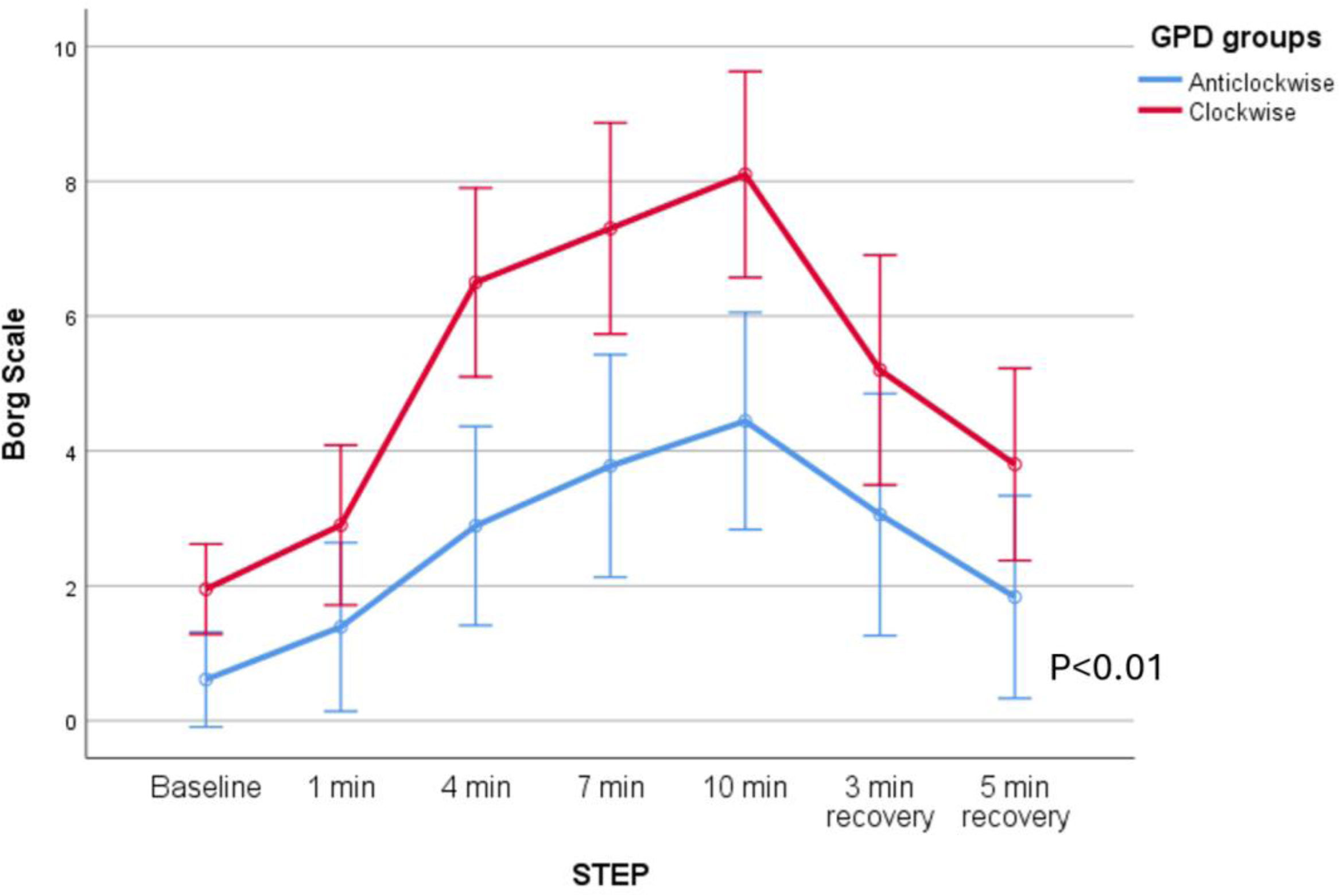
As shown in Fig. 2, patients with clockwise rotation (thorax ahead) had a higher degree of dyspnoea during unsupported exercise than patients with counterclockwise rotation (F=11.598, p<0.05, mean difference 2.53 Borg points, CI 95% 1–4.08, (β−1)=0.91), general linear model for repeated measures.
Borg Scale scores for both groups (patients with predominantly counterclockwise or clockwise rotation) during exercise with low-flow oxygen. As shown in the figure, patients with clockwise rotation exhibited higher dyspnoea levels compared to those with predominantly counterclockwise rotation (p<0.01, ANOVA for repeated measures).
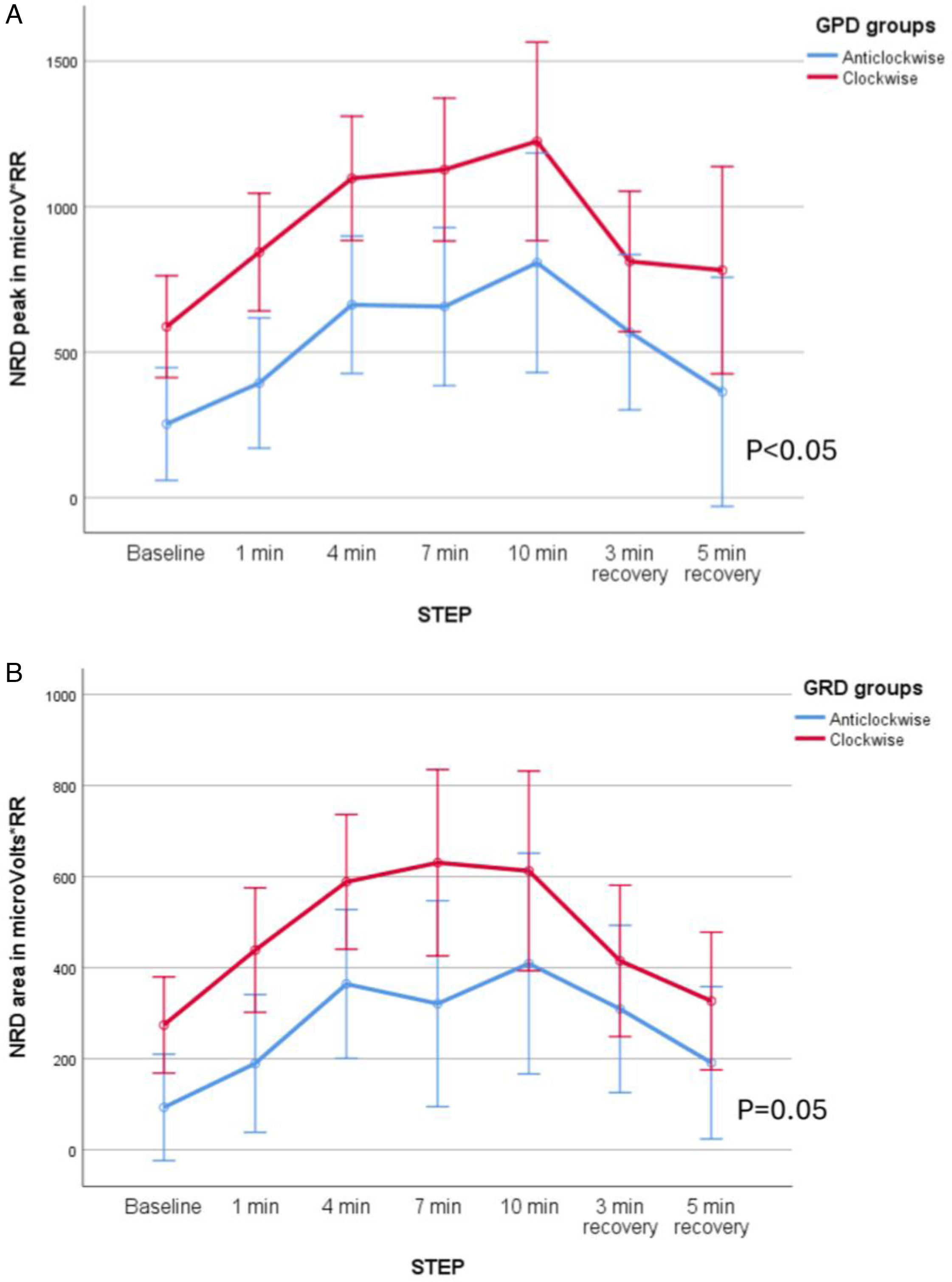
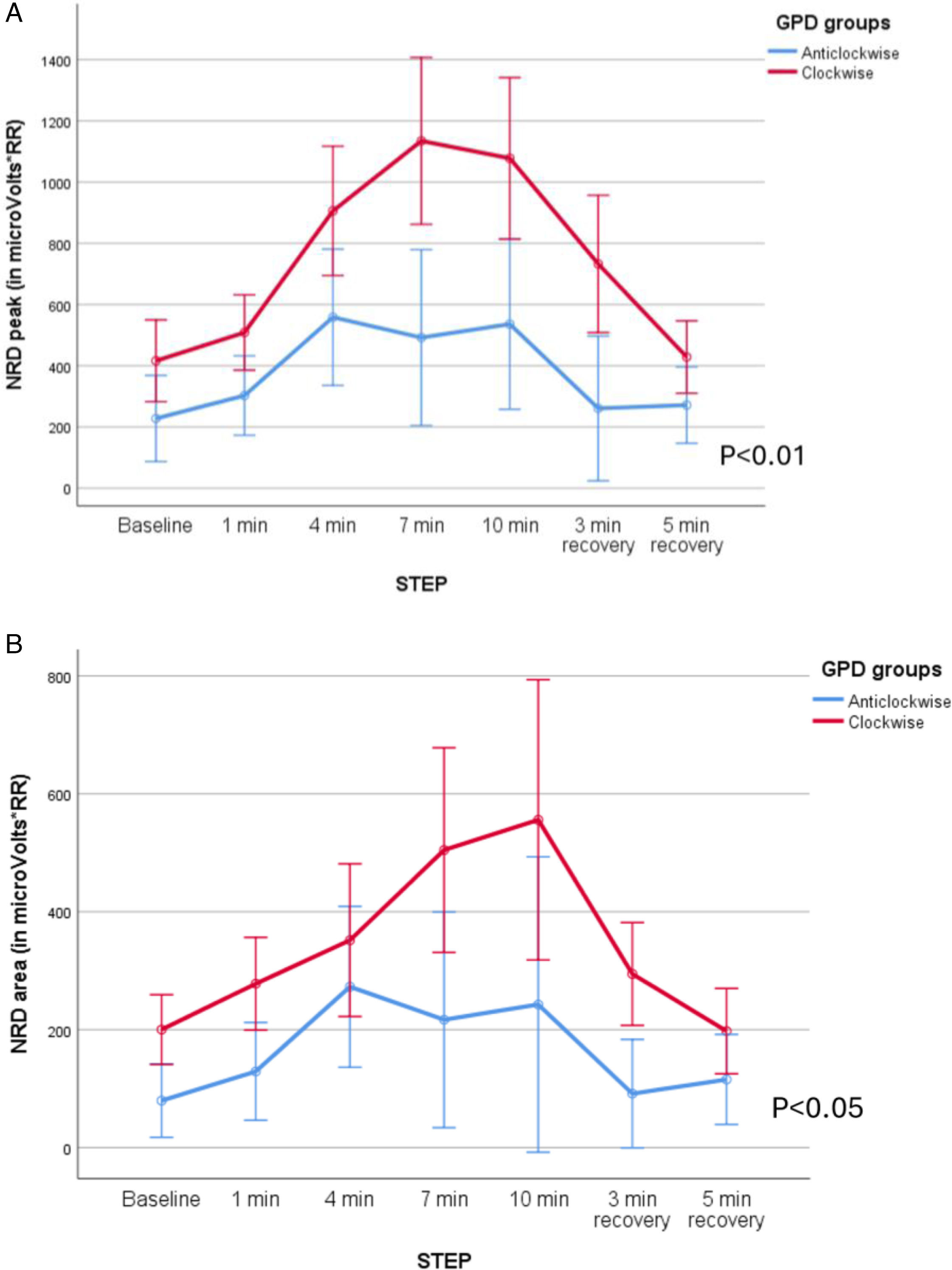
Patients with clockwise rotation also had higher NRD: peak RMS was higher (F=11.35, p<0.01, mean difference 456microV/RR, CI 95% 170.7–742.3, (β−1)=0.77) but not RMS area (F=4.35, p=0.05, mean difference 368microV/RR, CI 95% 264.7–472.3, (β−1)=0.44). When the subgroups based on the abdominal paradox were analyzed, patients with abdominal paradox showed higher NRD, both peak (F=27.49, p<0.01, mean difference 835microV/RR, CI 95% 716–955 (β−1)=0.99) and area (F=10.72, p<0.01, mean difference 423microV/RR, CI 95% 328–518, (β−1)=0.87). See Fig. 3A–D.
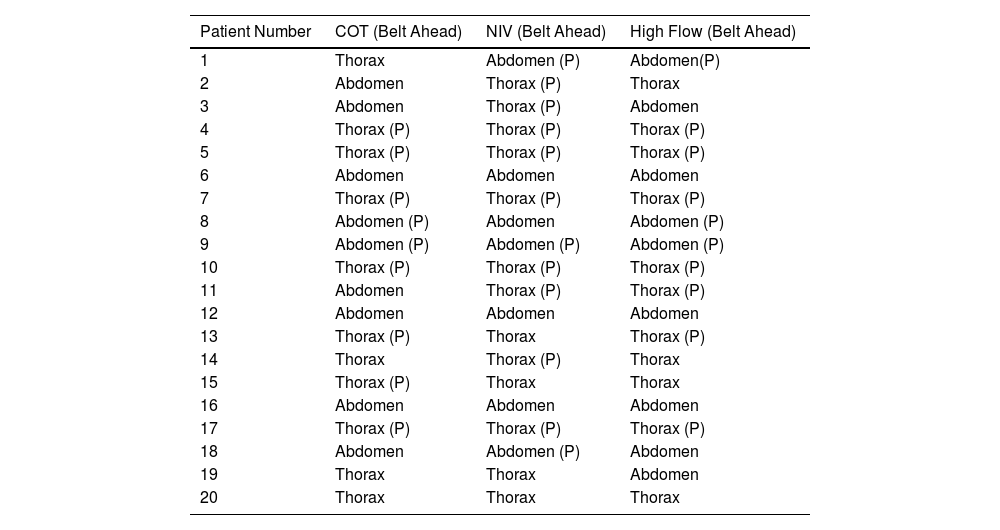
Exercise Under NIVUnder NIV 13 patients had clockwise rotation (9 with paradox) and 7 had predominantly counterclockwise rotation (3 with paradox), i.e. there were four patients who changed the direction of rotation (3 from counterclockwise to clockwise and one from clockwise to counterclockwise, see Table 3). No differences were observed in the degree of dyspnoea during exercise with NIV when the groups (clockwise or anticlockwise, abdominal paradox or no abdominal paradox) were compared. There were also no differences between groups in the ventilator parameters used to perform the test (inspiratory pressure, expiratory pressure and pressure support).
Predominant Direction of Rotation (Clockwise or Counterclockwise) for Each Individual Patient and Each Exercise Condition.
| Patient Number | COT (Belt Ahead) | NIV (Belt Ahead) | High Flow (Belt Ahead) |
|---|---|---|---|
| 1 | Thorax | Abdomen (P) | Abdomen(P) |
| 2 | Abdomen | Thorax (P) | Thorax |
| 3 | Abdomen | Thorax (P) | Abdomen |
| 4 | Thorax (P) | Thorax (P) | Thorax (P) |
| 5 | Thorax (P) | Thorax (P) | Thorax (P) |
| 6 | Abdomen | Abdomen | Abdomen |
| 7 | Thorax (P) | Thorax (P) | Thorax (P) |
| 8 | Abdomen (P) | Abdomen | Abdomen (P) |
| 9 | Abdomen (P) | Abdomen (P) | Abdomen (P) |
| 10 | Thorax (P) | Thorax (P) | Thorax (P) |
| 11 | Abdomen | Thorax (P) | Thorax (P) |
| 12 | Abdomen | Abdomen | Abdomen |
| 13 | Thorax (P) | Thorax | Thorax (P) |
| 14 | Thorax | Thorax (P) | Thorax |
| 15 | Thorax (P) | Thorax | Thorax |
| 16 | Abdomen | Abdomen | Abdomen |
| 17 | Thorax (P) | Thorax (P) | Thorax (P) |
| 18 | Abdomen | Abdomen (P) | Abdomen |
| 19 | Thorax | Thorax | Abdomen |
| 20 | Thorax | Thorax | Thorax |
P: Paradox.
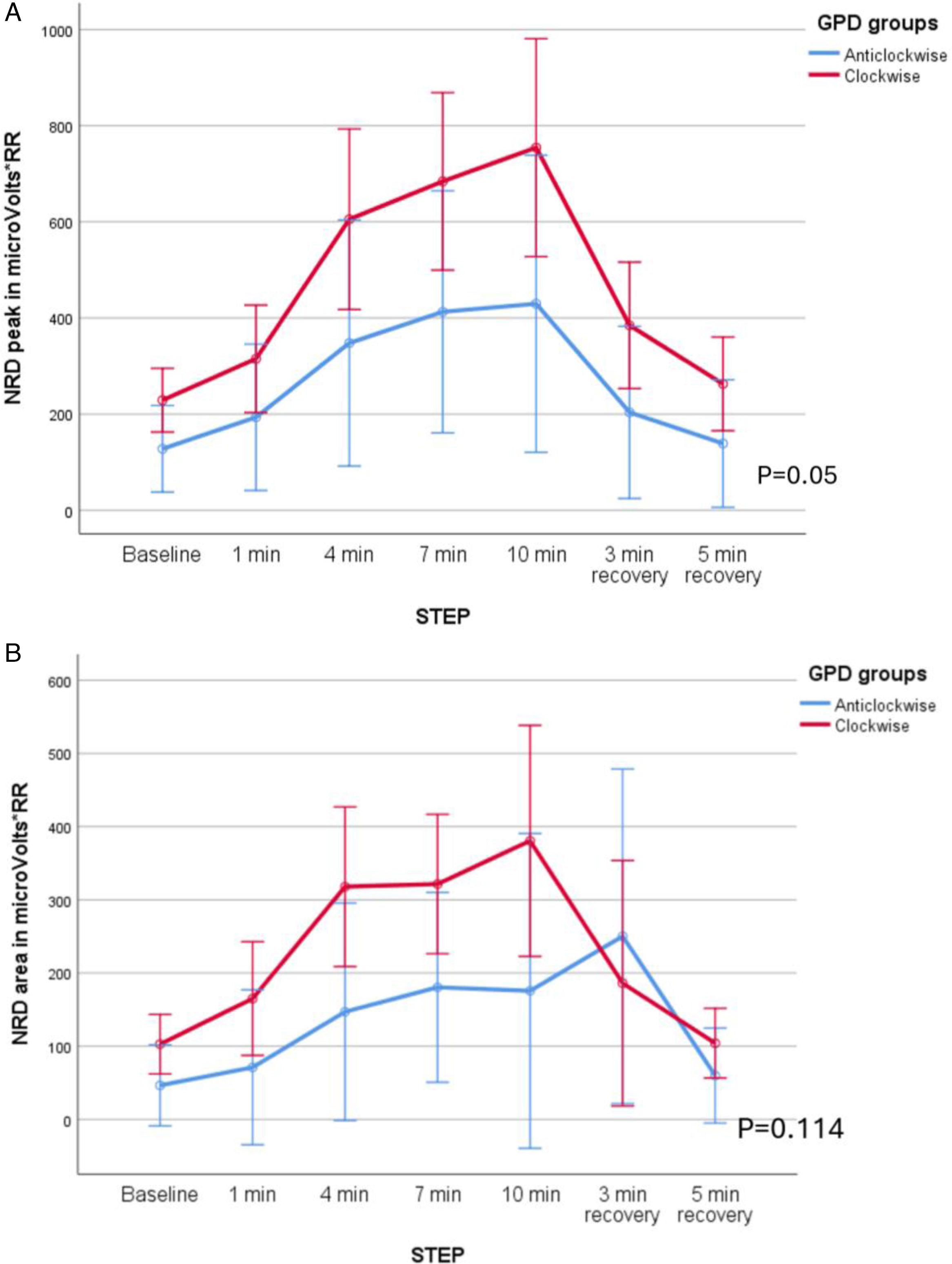
NRD was higher in patients with abdominal paradox both in peak (F=12.28, p<0.01, mean difference 406 microV/RR, CI 95% 324–488, (β−1)=0.91), and area (F=7.01, p<0.05, mean difference 199microV/RR, CI 95% 144–254, (β−1)=0.7), but no differences were found when the groups based on clockwise/counterclockwise were compared (Fig. 4A–D).
Exercise Under HFTEleven patients had clockwise rotation (7 with paradox) and 9 had predominantly counterclockwise rotation (3 with paradox). No differences were observed in the degree of dyspnoea as measured by the Borg scale during exercise with HFT when both groups (clockwise and anticlockwise) were compared.
NRD was higher in patients with clockwise rotation for the EMG peak (F=10.19, p<0.01, mean difference 555microV/RR, CI 95% 438–671, (β−1)=0.85) and area (F=7.31, p<0.05, mean difference 255microV/RR, CI 95% 184–326, (β−1)=0.72). When the group of patients with abdominal paradox were analyzed, they presented a higher NRD than patients without abdominal paradox: Peak: F=6.59, p<0.05, mean difference 660microV/RR, CI 95% 517–803, (β−1)=0.68 Area: F=13.19, p<0.01, mean difference 328microV/RR, CI 95% 254–402, (β−1)=0.92 (see Fig. 5A–D).
Comparing COT, NIV and HFTNo significant differences in the degree of TAA were observed when comparing the three interventions (COT, NIV and HFT). Three patients changed direction of rotation and development of paradox between interventions.
Dyspnoea, Respiratory Support and TAAComparing dyspnoea response to NIV and HFT in patients with clockwise or counterclockwise rotation showed no significant difference. However, patients with clockwise rotation showed a significant improvement in dyspnoea under NIV compared with COT (F=5.52, p<0.05, mean difference: 4.27, 95% CI: 3.58–4.95 (beta−1)=0.68). No improvement in dyspnoea was found between COT and HFT in any of the 2 different patterns of TAA. Figure E3 A to D (online supplement) reflect the effect in dyspnoea of COT vs NIV and HFT between those 2 patterns of TAA.
DiscussionTwo distinct patterns of TAA were identified during exercise in patients with advanced COPD: abdomen ahead (counterclockwise) and thorax ahead (clockwise). Notably, patients with severely impaired lung function exhibited an extreme clockwise pattern characterized by abdominal paradoxical breathing during exercise. This pattern was associated with greater hyperinflation, increased dyspnoea during COT exercise testing, and higher NRD, regardless of the type of respiratory support used (COT, NIV, or HFT). These findings suggest a compensatory activation of the parasternal muscles to counterbalance the diaphragm's inability to meet the increased respiratory demand.
Defining TAA as a delay between chest and abdominal movements highlights its role in ineffective respiratory mechanics, a subtype of dysfunctional breathing. It occurs in asthma, COPD, and neuromuscular diseases, with the most severe form being paradoxical (completely out-of-phase) movement.14–16 The abdominal paradox (abdomen moving inward during inspiration) is a classic sign of muscle fatigue, detectable via physical exam, and linked to severe diaphragmatic dysfunction, seen in 20% of COPD outpatients and 80% of acute respiratory failure cases. This pattern stands in clear contrast to the pattern observed in patients with air trapping but more preserved diaphragmatic function, who exhibit counterclockwise rotation with delayed thoracic expansion, known as “Hoover's sign.”17
It is difficult to compare our results with previous studies on TAA, as the calculation methods used in earlier research were not the same. For example, Alves et al. studied 22 COPD patients with an average FEV1 of 45% (much higher than in the present cohort) and evaluated synchrony at rest and during exercise. The reported phase angle values (not GPD) were 10° at rest and 22° during exercise, presumably indicating counterclockwise rotation.18 Chien et al. examined the impact of TAA on 6-min walk distance, but, like the study by Alves et al., they did not specify the leading compartment, and phase angle values remained below 35°, even in patients with severe COPD.15 Finally, Pereira et al. utilized RIP and OEP to analyze individuals with and without lung disease during exercise. Among COPD patients, the phase angle was predominantly negative and significantly narrower compared to the present study. Notably, previous studies did not establish a connection between RIP findings and clinical or respiratory function, which stands out as a key strength of the current study.6
The results of the present study should be understood from a pathophysiological perspective and may have implications for monitoring and designing exercise protocols: patients with worse lung function and more air trapping at rest often exhibit a well-defined clockwise pattern during exercise, suggesting fatigue or a loss of diaphragmatic efficiency caused by flattening and a decrease in the zone of apposition. Additionally, this pattern is associated with greater activation of the parasternal muscles and increased dyspnoea and may eventually contribute to evaluating the response to rehabilitation programmes. Interestingly, the most severe pattern (abdominal paradox) has not been previously reported or studied during exercise. Two reasons could be behind this fact: the way the RIP signal is analyzed, which may not show the presence of paradox, or the use of a recumbent cycloergometer, which may increase intra-abdominal pressure and make it harder for the diaphragm to contract.
One key aspect of the study is the response to non-invasive support techniques. A consistent decrease in TAA might be expected with NIV or HFT, but it's crucial to analyze these modes separately. No significant differences in TAA with NIV were found across the cohort, though responses varied widely (see Table 3). Patients with clockwise rotation may benefit most from exercise under NIV in terms of dyspnoea relief. Some patients showed an abdominal paradox with NIV before exercise, possibly due to excessive support (mean pressure support of 16cm H2O), which could worsen hyperinflation and lead to issues like deventilation syndrome.19
Despite the common practice of using NIV during exercise and its even recommendation in guidelines,20,21 there is a lack of objective data on the superiority of high-pressure support values (high-intensity ventilation) during exercise. In a randomized controlled trial, Schneeberger et al. found no significant difference in exercise capacity in patients receiving high-intensity ventilation.22 It remains to be demonstrated whether RIP monitoring can discriminate between patients who respond to high pressures and those who do not benefit from high pressures.
On the other way, the evidence supporting the benefits of HFT in the context of COPD rehabilitation programmes is limited.23 A recent meta-analysis found no significant difference in quality of life, exercise capacity and breathlessness between those who received HFT and those who received COT. In an experimental model, Adams et al.24 demonstrated that there was an increase in expiratory resistance that was directly proportional to flow and cannula size.
The results of the present study may have clinical implications for the management of patients with advanced COPD who are candidates for rehabilitation. Firstly, RIP can be used as a non-invasive monitoring method of the effects of rehabilitation programmes. Secondly, it can inform the titration of non-invasive respiratory support. In the event of paradoxical breathing (particularly abdominal) occurring in patients undergoing previously home NIV, the programmed parameters should be checked and readjusted if necessary. Ultimately, this approach can assist in identifying patients with severe clockwise rotation or abdominal paradox, who may require tailored rehabilitation programmes, particularly following an acute exacerbation.25
This study has relevant limitations. The sample size was limited and the study utilized a non-randomized design. It must be acknowledged that the conclusions of this study may not be applicable to other rehabilitation protocols, such as conventional cycloergometry or treadmill ergometry. Furthermore, the findings cannot be extrapolated to patients with less severe COPD or those with additional comorbidities, such as cardiovascular impairment.
In conclusion, the information provided by RIP allows the description of two distinct patterns of exertional TAA in patients with COPD. The thoracic advancement pattern is associated with increased NRD and greater dyspnoea. This finding may facilitate the personalization of exercise rehabilitation protocols and subsequent evaluation of their efficacy.
Authors’ ContributionsAll authors have significantly contributed to the entirety of the research process, including the conceptualization, data collection, analysis, and manuscript writing. Each author has approved the final version of the manuscript and agrees to be accountable for all aspects of the work.
Generative AINo use of artificial intelligence have been made in any phase of the work related this manuscript.
Funding of the ResearchPartially funded by a public research grant Instituto Carlos III (Madrid) Code: 18/1907 to Manel Luján Torné as principal investigator. This research did not receive any other specific grant from funding agencies in the commercial, or not-for-profit sectors
Conflicts of InterestThe authors declare that any of the following COI statements may be considered to influence directly or indirectly the content of the manuscript:
- •
Javier Sayas Catalán reports lecture fees from Philips, ResMed and Chiesi. Advisory board fees from Philips in the field of NIV, ResMed in the field of home care High flow therapy and Inogen in the field of oxygen therapy.
- •
Cristina Lalmolda reports lecture fees from Philips.
- •
Ana Hernández Voth reports fees from Chiesi and ResMed for lecture fees and research funding from Sanofi in the field of metabolic diseases.
- •
Marta Corral Blanco reports no competing interests or conflict of interests.
- •
Patrick Murphy reports lecture honoraria from Philips Respironics, Resmed, Fisher & Paykel, Chiesi, Genzyme.
- •
Laura González-Ramos reports lecture honoraria from Philips.
- •
Pablo Florez reports no conflict of interests.
- •
Berta Lloret-Puig reports no conflict of interests.
- •
Manel Lujan reports no conflict of interests.
Authors will like to acknowledge the work of Miguel Jimenez in editing the manuscript.