Lung cancer (LC) is the leading cause of death from cancer worldwide. More than 27,000 LCs are diagnosed annually in Spain, and most are unresectable. Early detection and treatment reduce LC mortality. This study describes surgical outcomes in a longstanding LC screening cohort in Spain.
MethodsWe conducted a retrospective study of surgical outcomes in a LC screening (LCS) program using low dose computed tomography (LDCT) since the year 2000. A descriptive analysis of clinical and radiological parameters, presence or absence of a preoperative diagnosis, pathological staging, morbidity, mortality, and survival was performed.
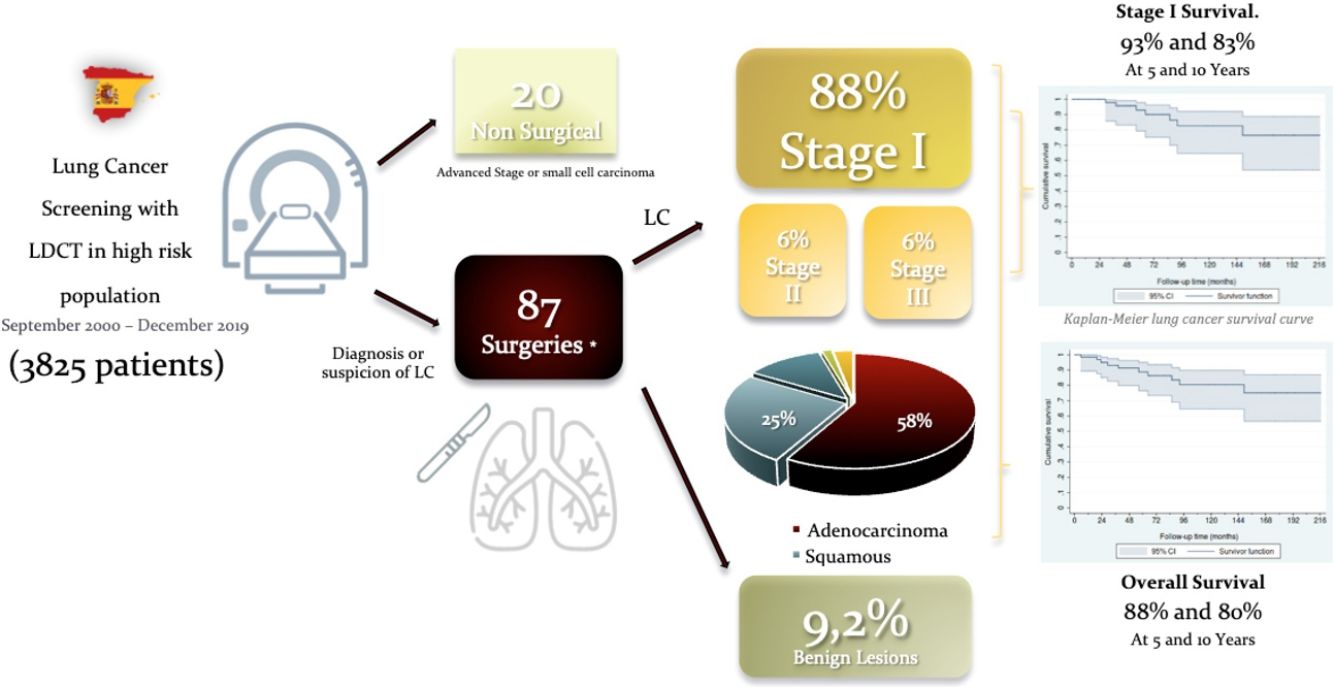
ResultsNinety-seven (2.5%) LC were diagnosed in 3825 screened. Twenty individuals with LC had no surgery due to advanced stage or small cell histology. Eighty-seven surgical procedures were carried out for suspected or biopsy proven LC, detected by LDCT. Most operated patients were male (57[85%]) aged 64±9.1 years. Nine patients underwent a second operation for a metachronous primary lung cancer. Mean tumor size was 15.2±7.6mm. Eight nodules were benign (9.2%). Lobectomy was performed in 56 cases (83.6%). Adenocarcinoma (n=39; 58.2%) was the most frequent histological type followed by squamous cell carcinoma (n=17; 25.4%). Fifty-nine (88%) tumors were in Stage I. Thirteen patients (15.4%) had 16 complications. The estimated survival rates at 5 and 10 years for stage I were 93% (95% CI: 79%–98%) and 83% (95% CI: 65%–92%), respectively.
ConclusionLung cancer screening was associated with excellent surgical outcomes with 5 and 10-year survival rates exceeding 90 and 80%, respectively.
El cáncer de pulmón (CP) es la principal causa de muerte por cáncer en todo el mundo. En España se diagnostican anualmente más de 27.000 CP y la mayoría son irresecables. La detección y el tratamiento tempranos reducen la mortalidad por CP. Este estudio describe los resultados quirúrgicos en una cohorte de cribado de CP de larga duración en España.
MétodosLlevamos a cabo un estudio retrospectivo de los resultados quirúrgicos de un programa de cribado de CP (CCP) usando tomografía computarizada de baja dosis (LDCT, por sus siglas en inglés) en marcha desde el año 2000. Se realizó un análisis descriptivo de los parámetros clínicos y radiológicos, presencia o ausencia de un diagnóstico preoperatorio, estadificación patológica, morbilidad, mortalidad y supervivencia.
ResultadosSe diagnosticaron 97 (2,5%) CP entre 3.825 sujetos cribados. Veinte personas con CP no se sometieron a cirugía debido a un estado avanzado de la enfermedad o a una histología de células pequeñas. Se llevaron a cabo 87 procedimientos quirúrgicos por sospecha de CP o CP demostrado mediante biopsia, detectados en la LDCT. La mayoría de los pacientes operados fueron varones (57 [85%]) de 64 años±9,1 años. Nueve pacientes se sometieron a una segunda operación por un cáncer de pulmón primario metacrónico. El tamaño medio del tumor fue de 15,2±7,6mm. Ocho nódulos fueron benignos (9,2%). Se realizó lobectomía en 56 casos (83,6%). El adenocarcinoma (n=39; 58,2%) fue el tipo histológico más frecuente seguido por el carcinoma de células escamosas (n=17; 25,4%); 59 (88%) tumores se encontraban en estadio I. Trece pacientes (15,4%) tuvieron 16 complicaciones. Las tasas de supervivencia estimadas a los 5 y 10 años para el estadio I fueron del 93% (IC 95%: 79 al 98%) y del 83% (IC 95%: 65 al 92%), respectivamente.
ConclusiónEl CCP se asoció con excelentes resultados quirúrgicos y con tasas de supervivencia a los 5 y 10 años superiores al 90 y al 80%, respectivamente.
Lung cancer (LC) is the leading cause of cancer-related deaths worldwide, with 1.8 million lung cancer deaths each year.1 Overall survival has not changed significantly in decades despite improvements in surgical treatment, chemotherapy, and radiotherapy.2 The poor survival rates are mainly a result of advanced stage at diagnosis.
Lung cancer screening (LCS) with the use of low dose computed tomography (LDCT) is effective in detecting LC in early stages and reducing mortality.3–6 The International Early Lung Cancer Action Program (I-ELCAP), showed in 2006 that 85% of LC cases diagnosed by LDCT were in stage I with an estimated survival of 88% at 10 years.7
In 2011 the National Lung Screening Trial (NLST), a randomized controlled trial, showed that LCS with LDCT reduces mortality in a high-risk population by 20% compared to screening with chest X-ray.8 The United Stated Preventive Services Task Force recommends screening with LDCT for individuals meeting NLST inclusion criteria since 2013.9
Implementation of LCS guidelines in the United States is ongoing. Widespread screening in Europe 10 was precluded until the result of the Dutch-Belgian NELSON study were published. This publication has now finally been published 11 reported that LCS can significantly reduce deaths from lung cancer at 10 years by 24% in men and up to 33% in women compared to control.
Despite such promising results, screening skeptics continue to argue that LCS may cause harm by leading to invasive procedures in asymptomatic individuals with false positive screening findings.12 Our group has published several papers analyzing different aspects related to this lung cancer screening cohort.13–16 The work presented here is an analysis from a surgical point of view of the longest running lung cancer screening program in Europe, at the Clínica Universidad de Navarra, a program that is part of the I-ELCAP consortium since its inception in 1999.3,15
Materials and MethodsThis is a retrospective descriptive analysis of a prospectively recruited cohort. Study subjects enrolled in the Pamplona International Program for Early Detection of Lung Cancer with LDCT (P-ELCAP). Patients undergoing surgery based on screening findings are included in this study. Recruitment began in September of 2000 and is ongoing. Patient follow up is based on the I-ELCAP protocol, details of which are available online at www.ielcap.org. Inclusion criteria at our center include age≥40, and a smoking history of ≥10 pack-years. Subjects must be asymptomatic. Those with a history of cancer diagnosed within the previous 5 years are excluded. Baseline LDCT and spirometry are performed in all cases, and periodic follow up with LDCT is recommended thereafter. Subjects with abnormal LDCT findings are managed according to the I-ELCAP protocol.17 Briefly, for positive results on the baseline LDCT (noncalcified nodules ≥5mm) additional diagnostic studies are recommended depending on the size and characteristics of a given nodule, and may include PET-CT scanning, antibiotic treatment with follow up LDCT at 1–3 months, bronchoscopy, transthoracic fine needle aspiration, or surgical biopsy. Subjects with a negative LDCT (without pulmonary nodules or with non-calcified nodules <5mm) are scheduled for an annual visit. Annual LDCT is considered abnormal when a new nodule of any size is found or when a previously identified nodule grows, prompting ancillary studies. Prior to pulmonary resection, the mediastinum was further assessed, endobronchial ultrasound (EBUS), through endoscopic ultrasound (EUS) or mediastinoscopy. In all cases, a multidisciplinary team made up of pathologists, oncologists, pulmonologists, radiologists and thoracic surgeons decided whether surgical or oncological interventions were required.
For the purpose of the current study, the following parameters were considered: demographics, preoperative tests, surgical approach, type of lung resection, immediate and late post-surgical complications, anatomopathological results, stages, and survival.
Respiratory health status was determined by smoking status and the presence or absence of chronic obstructive pulmonary disease (COPD), defined as postbronchodilator FEV1/FVC<70%, the presence of emphysema on LDCT, or both.
Staging of LC and definition of a metachronous tumor were defined according to the 8th edition of the international TNM classification.18 Mediastinal nodal dissection (MLND) was performed according to the European Society of Thoracic Surgeons guidelines,19 i.e., at least three mediastinal nodal stations, including the subcarinal lymph node, were excised. Mediastinal lymph node sampling (MLNS) was defined just as the removal of one or more lymph nodes, guided by preoperative or intraoperative findings at the surgeon's discretion.
A false positive of the screening process was considered when a patient underwent surgery after all pre-surgical diagnostic tests (including LDCTs) could not rule out lung cancer, and the pathology of the resected lesion was consistent with a benign diagnosis.
Data AnalysisQuantitative data are presented as mean and standard deviation (SD). Categorical data were described using absolute and relative frequencies.
Lung cancer prevalence and incidence rates were calculated according to whether the diagnosis was based on the results of the baseline evaluation or on one of the follow-up screening rounds, respectively. Operative mortality included patients who died within 30 days. Medical records were reviewed to obtain mortality data on patients with lung cancer, and a Kaplan–Meier survival curve was constructed. All statistical analyses were performed using Stata/IC 12.1 (StataCorp. 2011. Stata Statistical Software: Release 12. College Station, TX: StataCorp LP).
Ethics ApprovalThe University of Navarra's ethics committee approved the study protocol and all subjects signed an informed consent prior to enrollment in LCS.
ResultBetween September 2000 to December 2019, 97 LC (2.5%) were diagnosed in 3825 participants screened. Twenty patients had no surgery due to advanced stage or small cell histology and were referred to chemo-radiotherapy or SBRT. Eighty-seven surgeries were performed in 75 patients for suspected LC. When a pre-operative biopsy was not feasible, we used wedge resection and frozen section as diagnostic tools. In case of lung cancer, the resection was performed during the same procedure, except in three patients, who had a wedge resection followed by a second surgery to complete the resection, due to an uncertain intraoperative diagnosis, once the definitive pathology results were known. Only 14 patients had a non-surgical preoperative diagnosis of LC obtained by CT-guided fine needle aspiration cytology (FNAC) (7), bronchoscopy (4), EBUS (1), and EUS (2) (Table 1).
Preoperative Diagnosis Technique.
| Surgical Patients | Cases (N=87) |
|---|---|
| Preoperative pathological diagnosis obtained by: | 17 (19.5%) |
| CT guided FNAC | 7 |
| Bronchoscopy | 4 |
| EBUS (Endobronchial ultrasound) | 1 |
| EUS (Endoscopic ultrasound) | 2 |
| Surgical wedge resectiona | 3 |
Nine patients underwent a second operation for a metachronous primary lung cancer. One patient had a synchronous tumor resected in the same lobe. Eight (9.2%) patients were false positive of the screening process and all of them had diagnostic wedge resections (Table 2). One individual had 2 surgeries for a LC and a benign lesion.
Confirmed Surgical Benign Diagnosis.
| Benign Lesions/Screening Process False Positives | Cases (N=87) |
|---|---|
| Histopathology, n (%) | 8 (9.2%) |
| Pulmonary fibrosis: N (%) | 3 (37.5%) |
| Lymph node: N (%) | 2 (25%) |
| Sclerosing haemangioma: N (%) | 1 (12.5%) |
| Atypical adenomatous hyperplasia: N (%) | 1 (12.5%) |
| Chronic inflammatory process | 1 (12.5%) |
To maintain data independence, only the therapeutic surgeries and the first surgery of the metachronous cases (n=67) were taken into consideration for descriptive and statistical analysis in LC patients.
Fifty-seven (85%) patients were male with a mean age of 67±9.1 years. Mean tumor size was 15.2±7.6. Seven patients (10.5%) had COPD, 21 (31.3%) had emphysema, 28 (41.8%) had both, and 11 (16.4%) had neither of those key respiratory comorbidities (Table 3).
Patient Characteristics.
| Patients Characteristics | N=67 |
|---|---|
| Males, n (%) | 57 (85%) |
| Females, n (%) | 10 (15%) |
| Age, Mean (SD) | 34 (9.1) |
| BMI, Mean (SD) | 26.9 (4.7) |
| Pack/years, Mean (SD) | 53 (24) |
| FVC, Mean (SD) | 3.9 (0.9) |
| FEV1, Mean (SD) | 2.6 (31.3) |
| FEV1/FVC, Mean (SD) | 67 (9.4) |
| Health status, n (%) | |
| No COPD nor emphysema | 11 (16.4%) |
| COPD (only) | 7 (10.5%) |
| Emphysema (only) | 21(31.3%) |
| COPD and emphysema | 28 (41.8%) |
Surgical approaches included 44 posterolateral (65.7%), 4 anterolateral (6%), and 3 axillary thoracotomies (4.5%), 1 robotic-assisted thoracic surgery (RATS) (1.5%), and 15 video-assisted thoracoscopic surgeries (VATS) (22.4%).
Surgical lobectomy was performed in 56 cases (83.6%). Sublobar resections included; 3 anatomical segmentectomies (4.5%) for tumors measuring less than 1cm and clinical stage IA in which margins were confirmed intraoperatively to be >1cm, and 7 (10.5%) wedge resections in patients with limited lung function. One pneumonectomy (1.5%) was performed for a central tumor. Thirty-seven (55.2%) patients underwent MLND and 30 (44.8%) had MLNS. Adenocarcinoma (n=39, 58.2%) was the most frequent histological type followed by squamous cell carcinoma (n=17, 25.7%) (Table 4).
Surgical Lung Cancer Patients’ Characteristics.
| N=67 | |
|---|---|
| Method of resection, n (%) | |
| Posterolateral thoracotomy | 44 (65.7%) |
| Anterolateral thoracotomy | 4 (6.0%) |
| Axillary thoracotomy | 3 (4.5%) |
| Video-assisted thoracoscopic surgery | 15 (22.4%) |
| Robotic-assisted thoracic surgery | 1 (1.5%) |
| Type of resection,n(%) | |
| Sublobar | |
| Segmentectomy | 3 (4.5%) |
| Wedge resection | 7 (10.4%) |
| Lobectomy | 56 (83.6%) |
| Pneumonectomy | 1 (1.5%) |
| Lymph node resection,n(%) | |
| Mediastinal lymph node dissection | 37 (55.2%) |
| Mediastinal lymph node sampling | 30 (44.8%) |
| Histopathology,n(%) | |
| Adenocarcinoma | 39 (58.2%) |
| Squamous | 17 (25.4%) |
| Large cell | 8 (11.9%) |
| Small cell | 1 (1.5%) |
| Typical carcinoid | 1 (1.5%) |
| Atypical carcinoid | 1 (1.5%) |
Sixteen complications were reported in 13 patients, including; persistent air leak (5), atrial fibrillation (2), and acute urinary retention (4), re-intervention due to bleeding (3), pneumonia (1), and empyema (1). There were no complications in the false positives of the screening process patients. One patient (1.2%) died from postoperative acute respiratory distress syndrome following a second pulmonary resection surgery due to a confirmed contralateral Stage II metachronous tumor.
Of the 67 patients, 40 (59.7%) were diagnosed in the baseline screening of which 87.5% were in stage I. Twenty-seven (40.3%) patients were diagnosed in annual screenings, 88.9% of which were in stage I (Table 5).
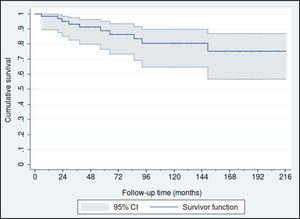
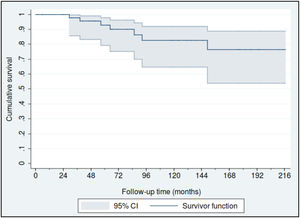
Fifty-nine patients (88.1%) were diagnosed in Stage I, 4 in Stage II (6%) and another 4 in stage III (6%). The estimated overall survival rates at 5 and 10 years were 88% (95% CI: 77%–95%) and 80% (95% CI: 65%–90%), respectively (Fig. 1), and the 5 and 10 year survival rates for stage I patients were 93% (95% CI: 79%–98%) and 83% (95% CI: 65%–92%), respectively (Fig. 2).
DiscussionOur study confirms previous findings regarding favorable surgical outcomes in the LCS setting, with overall five and 10-year survival rates of 88% and 80%, respectively. The majority of subjects in this cohort underwent lobectomies via thoracotomy, a surgical approach that may become obsolete as sublobar resections and minimal invasive techniques become more prevalent in the LCS setting. Nevertheless, the surgical treatment of lung cancer in early stages and the extent of lung resections, with its implications for survival, still requires extensive research. In our series, a lobectomy was considered the “gold standard” of pulmonary resection for patients with adequate lung function and a confirmed diagnosis of malignancy.
Most retrospective studies have shown that VATS segmentectomy may be a valid procedure lung cancer patients in clinical stage I,20–23 with lower complication rates and shorter hospital stays, and no differences in terms of overall survival. Two randomized controlled trials in progress will attempt to answer these questions (CALGB 140503 and JCOG0802/WJOG4607L).24,25
Only 22.4% of the surgeries in our study were VATS, mainly due to the slow implementation of VATS in our center. It has been shown that, as compared to open classical thoracotomy, VATS is associated with a significant reduction in perioperative morbidity and mortality.26 In a randomized trial, Bendixen et al. observed that when compared to antero-lateral thoracotomy, VATS is associated with less postoperative pain and better quality of life during the first year after surgery.27 Preliminary results of the VIOLET trial,28 a randomized multicenter trial in the United Kingdom led by Dr. Eric Lim comparing the performance and morbidity of VATS with open thoracotomy, showed that patients who received VATS had a significant reduction in overall in-hospital complications and stayed in hospital one day less compared to patients who received open surgery. That notwithstanding, oncologic outcomes were similar. Therefore, it appears that minimal invasive approaches may well become the surgery of choice for LC resections and even moreso in the context of LCS.
In our series, 55.2% of the subjects underwent systematic lymph node dissection. Controversy persists regarding the optimal extent of lymph node dissection in early stage LC. European Thoracic Surgery Society guidelines recommended a systematic dissection of nodes at all stages in 2006 to ensure complete resection and adequate staging.19 Lobe specific nodal dissection is considered acceptable for peripheral T1 lesions if hilar and interlobar nodes are negative at intra operative frozen section. A similar recommendation was proposed in 2010 by the British Thoracic Society.29 Subsequently, in a randomized controlled trial comparing mediastinal lymph node dissection (MLND) vs. sampling after negative side-specific hilar and mediastinal lymph node sampling, Darling et al. demonstrated that MLND did not improve survival nor did it decrease the incidence of local or regional recurrence of patients with early-stage non small cell LC.30
In our cohort, an overall positive effect on survival rates was observed in the MLND group (log-rank-test P=.04). However, when we split the data set according to early (I) and more advanced stages (II and III), the difference between MLND and MLNS only held true for the former. There is inconsistency among published reports in early stage NSCLC, which could be related to tumor size.31,32 Lymphadenectomy and the extent of lung resection in LCS early stage disease should benefit from further prospective studies and larger sample sizes.
Ongoing studies, like IELCART (Initiative for Early Lung Cancer Research on Treatment)33 in which our institution participates, aim to advance knowledge regarding the optimal surgical treatment of patients with early stage LC. Key variables include; the type of resection, standardization of margin measurements, extent of lymphadenectomy, and alternative therapeutic approaches (SBRT or watchful waiting), as well as the impact on quality of life, recurrence rates, and long-term survival.
Interestingly, after the diagnosis and surgical treatment of a first LC we found a significant number of patients with a second metachronic primary tumor that met the Martini-Melamed and the IASLC criteria.18 This raises an important issue. Patients with lung cancer are at a very high risk of developing additional lung cancers. In our cohort, 9 patients had more than one metachronous cancer. Since most patients with lung cancer also have co-existing lung disease, such as COPD and emphysema,34 a conservative lung-sparing surgical approach may be important since additional resections may be in store for a given LCS patient. In addition, this increased risk for subsequent lung cancers underlines the need for continuing lung cancer screening in patients who undergo resection.35
On the other hand, one of the main obstacles of LCS is the very nature of an early detection program, in which the suspicious lesions are almost always small. They may not be anatomically located in peripheral or accessible areas and/or have a partially solid consistency, which would make it difficult, or even impossible, to obtain a preoperative diagnosis via transthoracic or endoscopic needle aspiration. Therefore, it is not unusual for surgery to be both diagnostic and therapeutic in this setting.
Our series demonstrated certain limitations to obtain a preoperative diagnosis with only 16% of surgical patients. Those indeterminate cases, which require diagnostic surgery, demand a biopsy of the suspicious lesion in order to allow an adequate intraoperative diagnosis and by consequence, the definition of the extent of the curative pulmonary resection. The reason why most nodules are undiagnosed prior to surgery is likely the small size and location. This also entails difficulties for intraoperative diagnosis due to the limitations for the surgeon to see and feel the lesions in the context of minimal invasive surgery.
Therefore, identifying and locating these lesions has become a veritable challenge, which has created a surge in different preoperative marking alternatives. This is why preoperative marking of lesions is a tool which every LCS Program must count on.36,37
Having a high proportion of undiagnosed patients making it to the operating room, together with the false-positive rate described, suggests that proper risk assessment, compliance with a correct diagnostic algorithm, and a consensus decision by an expert multidisciplinary committee are key in LCS. Unnecessary invasive procedures must be limited in order for LCS to succeed.
The results in this cohort, with 9.2% of false positives of the screening process that underwent surgery, coincide with the results of I-ELCAP.
Several strategies have been suggested to decrease it. Firstly, a better selection of the inclusion criteria, which would lead to a more precise definition of high-risk candidates, should be considered. Standardized and structured reporting systems such as Lung-RADS38 or the I-ELCAP management system17 should be used. Refined diagnostic algorithms determined under consensus and scientific evidence should be adapted, and prediction tools for risk analysis should be established.39,40 Finally, well validated radiological techniques, such as the volumetric study of indeterminate pulmonary nodules,41 artificial intelligence and radiomic lung nodules prediction models,42,43 should all be encouraged. These, together with the management by an expert multidisciplinary team, have demonstrated their effectiveness in minimizing surgical intervention for benign disease, minimizing the number of missed curable LC. Even so, false positives would be difficult to avoid completely. The evidence of surgical procedures for benign disease in LCS with LDCT varies from 0% to 33%, with an average of 18%.44 Wilson and colleagues evaluated 3642 participants in the PLUSS 45 study using an internal protocol. Eighty-two (2.3%) underwent surgical procedures, 28 of which (34%) had benign disease. The study investigators cited “an apparent generalized bias toward aggressive intervention” for indeterminate pulmonary nodules. The DLCTS (Danish Lung Cancer Screening Trial) group reported 12% of false-positive surgeries.36 Our results show that following validated management protocols, such as that of IELCAP, can minimize the number of individuals who undergo surgery for a benign lesion. Albeit, of those who unfortunately still underwent surgery, none showed any consequential complications.
We agree with current European guidelines suggesting that LCS should guarantee comprehensive quality of care and be led by an expert multidisciplinary team that provides high quality, timely, continuous and comprehensive information on the benefits and risks of screening. Informed decision-making eliminates misperceptions and reduces the psychological burden related to diagnostic and therapeutic interventions. The multidisciplinary team should include surgeons with experience in minimally invasive procedures and should be equipped with technical and/or technological tools that allow for intra-operative localization of small nodules. Furthermore, we must not forget that every LCS program must include smoking cessation and should emphasize the importance of adherence to the program with a commitment to annual screening even in the face of prior negative results, a limitation already described by programs like ours.15,46,47
One potential limitation of this study is that it is based on data from a single center; therefore, our population, resources, and staffing characteristics may limit the generalizability of our results. However, we believe that surgery for lung cancer is quite standardized across Centers and that similar results will probably be achieved by others.
In summary, our results show that lung cancer screening is effective in detecting lung cancer at early stages, and that the majority of patients achieve long term survival after surgical resection. Ongoing research trials will help determine what the optimal surgical approach is for these patients.
Funding SourceThe authors received no specific funding for this work.
Conflict of InterestsThe authors declare that they have no conflict of interests.