A 68-years-old female patient was admitted in Neurology with complaints of dysphagia, rigidity, paresthesias and spasms on her left inferior limb for 6 days. She was a former smoker, had a polyarthritis in treatment with hydroxychloroquine and had been diagnosed several years ago of chronic obstructive pulmonary disease.
During hospitalization, ordinary laboratory parameters were within normal range except for high CPK (1.866U/L). Chest X-rays, body positron emission tomography–tomography scan, analysis of cerebrospinal fluid and brain and spine magnetic resonance image were normal. Electromyography was diagnostic for stiff-person syndrome and serum antigliadin antibodies were strongly positive.
Symptomatic treatment was initiated including muscle relaxants and antispastic agents. However, although limb stiffness slightly improved, patient presented dyspnea and dysphagia and she underwent aspiration pneumonia and was admitted to the Intensive Care Unit with high needs of oxygen and noninvasive ventilation (NIV). Neurological treatment was intensified adding baclofen, levetiracetam, valproic acid and finally progressing to immunoglobulin and high doses of intravenous corticosteroids, which led to a remarkable respiratory improvement, being discharged to hospitalization ward 8 days later.
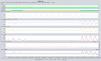
After a few days, patient suffered a new respiratory failure episode. However, despite initiation of NIV and exhaustive adjustment, the patient experienced progressive worsening (pH 7.19 and PaCO2 100mmHg), detecting in the ventilator graphics a decrease of inspiratory flow with absence of thoraco-abdominal movement, suggesting upper airway obstructive events with reduced neural drive (Fig. 1).
Stiff-person syndrome (SPS) spectrum disorders (SPSSD) is a rare heterogeneous disorders that cause spasms and rigidity in legs and axial musculature. SPSSD has often an autoimmune origin (70%) associated with anti-glutamic-acid-descarboxylase (GAD) and anti-gliadin (GlyR), usually co-existing with other autoimmune disorders (mainly Type 1 Diabetes).1,2 In 20% of cases has a paraneoplastic origin, usually related to breast and lung cancer, as well as Hodgkin lymphoma.1 Diagnostic delay is frequent because of its rareness. Initial treatment is symptomatic, considering immunosuppressive therapy with corticosteroids in the absence of improvement or if the complications are life-threatening.3 Patients not responsive to or unable to tolerate corticosteroids can be treated with intravenous immunoglobulin therapy or Rituximab.4 Plasmapheresis is also described with variable results.1
Approximately half of patients with SPS experience dyspnea which increases as more body regions are involved.1 Respiratory muscle involvement can occur from the onset or during the disease. A restrictive spirometry pattern has been describe in these patients,2 although an obstructive pattern has also reported related to comorbid condition.1 SPSD can be complicated with apnea episodes than can be linked with acute respiratory failure and sudden death.1–5 Underlying physiopathology of respiratory failure is controversial, two main mechanisms have been proposed: muscle stiffness and paroxysmal respiratory muscle spasms and paroxysmal hyperactivity of the autonomous nervous system2; both situations are caused by an impairment in GABAergic or glycine inhibitor pathways mediated by anti-GAD autoantibodies inhibiting GABA synthesis.2
Acute ventilatory failure with apnea episodes is a life-threatening complication of SPS; NIV is useful in the management of acute or chronic respiratory failure when bulbar function is not severely affected.5 Intubation is indicated if noninvasive measures fail.
Conflict of interestsThe authors state that they have no conflict of interests.