CURRENT SITUATION AND LONG-TERM CONSEQUENCES OF COVID-19 INFECTION
More infoOn March 11, 2020, the World Health Organization declared Coronavirus Disease 2019 (COVID-19) a pandemic. Till now, it affected 452.4 million (Spain, 11.18 million) persons all over the world with a total of 6.04 million of deaths (Spain, 100,992). It is observed that 75% of hospitalized COVID-19 patients have at least one COVID-19 associated comorbidity. It was shown that people with underlying chronic illnesses are more likely to get it and grow seriously ill. Individuals with COVID-19 who have a past medical history of cardiovascular disorder, cancer, obesity, chronic lung disease, diabetes, or neurological disease had the worst prognosis and are more likely to develop acute respiratory distress syndrome or pneumonia. COVID-19 can affect the respiratory system in a variety of ways and across a spectrum of levels of disease severity, depending on a person's immune system, age and comorbidities. Symptoms can range from mild, such as cough, shortness of breath and fever, to critical disease, including respiratory failure, shock and multi-organ system failure. So, COVID-19 infection can cause overall worsening of these previous respiratory diseases, such as asthma, chronic obstructive pulmonary disease (COPD), interstitial lung disease, etc. This review aims to provide information on the impact of the COVID-19 disease on pre-existing lung comorbidities.
El 11 de marzo de 2020, la Organización Mundial de la Salud declaró la enfermedad por coronavirus 2019 (COVID-19) como pandemia. Hasta ahora, ha afectado a 452,4 millones (en España, 11,18 millones) de personas en todo el mundo, con un total de 6,04 millones de muertes (en España, 100.992). Se observa que el 75% de los pacientes hospitalizados por COVID-19 tienen al menos una morbilidad concomitante a esta enfermedad. Se ha demostrado que las personas con enfermedades crónicas subyacentes tienen más probabilidades de contraerla y enfermar gravemente. Los individuos con COVID-19 con antecedentes de trastorno cardiovascular, cáncer, obesidad, enfermedad pulmonar crónica, diabetes o enfermedad neurológica tienen el peor pronóstico, y son más propensos a sufrir el síndrome de dificultad respiratoria aguda o neumonía. La COVID-19 puede afectar al sistema respiratorio de diversas maneras y en un espectro de gravedades de la enfermedad, dependiendo del sistema inmunitario de la persona, la edad y las comorbilidades. Los síntomas pueden ir desde los más leves, como tos, dificultad para respirar y fiebre, hasta los más graves, como insuficiencia respiratoria, shock y fallo multiorgánico. Así, la infección por COVID-19 puede generar un empeoramiento general de estas enfermedades respiratorias previas, como asma, enfermedad pulmonar obstructiva crónica, enfermedad pulmonar intersticial, etc. Esta revisión tiene como objetivo proporcionar información sobre el impacto de la enfermedad por COVID-19 en las comorbilidades pulmonares preexistentes.
The coronavirus disease 2019 (COVID-19) pandemic, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has led to high morbidity and mortality worldwide. SARS-CoV-2 predominantly affects the lung, but it can also affect other organs such as the brain, heart, and gastrointestinal system. It is observed that 75% of hospitalized COVID-19 patients have at least one COVID-19 associated comorbidity. Although the mean age of COVID-19 infected subjects has changed from older to younger patients1–3 based on current evidence, risk factors for developing and having poor prognosis of COVID-19 in adults are smoking habits, demographic factors, such as older age, male sex, and ethnicity, and the presence of underlying diseases such as cardiovascular diseases, hypertension, and chronic obstructive pulmonary disease (COPD).4–6 Being a younger children and specific comorbidities such as obesity or being a smoker is at higher risk for infection and potentially more severe consequences of COVID-19. In addition to these factors, changes in laboratory indices and pro-inflammatory cytokines, as well as possible complications, could indicate the progression of COVID-19 into a severe and critical stage.7–11
An important percentage of subjects has important clinical, functional or radiological sequelae (long-term COVID-19 syndrome) months after the acute infection, especially those who suffered from a severe pneumonia admitted to the intensive care unit (ICU).12–16 Finally, many treatments both pharmacological (especially antivirals, immunomodulators and anti-inflammatories) and non-pharmacological treatments has been used during this pandemic era at different moments and severities of the disease, sometimes with controversial results.17–24
On the other hand, what effects does COVID-19 disease have on previous lung diseases? After COVID-19 disease recovery time appears to be around 2 weeks for mild infection and 3–6 weeks for severe disease; however, this is variable and depends on a patient's pre-existing comorbidities in addition to illness severity. A major issue with COVID-19 is with gas exchange in the alveolus. Usually, there is a very tight connection between the alveolar epithelium (type-1 cells) and the capillary. COVID-19 infects AT2 cells, kills them and floods the alveolus. In addition, there is evidence for microthrombosis,25 which may block the vascular side. COVID-19 can cause permanent lung damage with higher risk of long-term complications (chronic inflammation has been considered as the main cause of pulmonary fibrosis and may lead to epithelial damage and fibroblast activation).26 This review aims to provide information on the impact of the COVID-19 on pre-existing lung comorbidities.
Obstructive sleep apneaJust as sleep apnea syndrome (OSA) is a risk factor for pneumonia,27 observational studies show that OSA can be a risk factor for both COVID-19 infection and unfavorable outcome.28
Labarca et al.29 conducted a case–control study among patients with acute respiratory distress syndrome (ARDS) secondary to COVID-19 and mild or moderate disease, showing that, in surviving patients, the prevalence of undiagnosed OSA was statistically significant compared to patients with mild or moderate disease. After adjusting for other confounders, OSA was independently associated with ARDS. Moreover, undiagnosed OSA presented more pulmonary sequelae in the medium term, in addition to being associated with variables such as male gender, ARDS, and total days on invasive mechanical ventilation (IMV).
In the CORONADO observational study, untreated OSA was an independent risk factor for 7-day mortality, with an odds ratio (OR) of 2.8.30 In a study that included 46 patients hospitalized for COVID-19, OSA was diagnosed in 75% of the sample,31 and another observational study showed that patients with OSA presented an OR of 1.53 for mortality and 1.29 for ICU admission.32
A recent meta-analysis33 of a total of 21 studies with 54,276 COVID-19 patients showed that OSA was associated with composite poor outcome and its subgroup which comprised of severe COVID-19, ICU admissions, the need for IMV and mortality.
There is an interaction between obesity and OSA, and in this sense, a greater number of obese patients required admission to the ICU and up to 7 times more IMV, with a body mass index (BMI)≥3534,35 being an independent factor of mortality.36
OSA and COVID-19 are proinflammatory states, so prior chronic systemic inflammation from untreated OSA would be a predisposing factor.37 In addition, sleep fragmentation and chronic intermittent hypoxia can trigger an inflammatory response and sympathetic activation.38 Both COVID-19, obesity, and OSA increase hypoxia-inducible factor 1-alpha levels, which increase the cytokine storm39 that occurs in COVID-19 pneumonia patients and in those with subsequent multi-organ failure.40
Hypoxemia and altered hemodynamics in OSA can precipitate a pro-coagulant state, which could further accentuate coagulopathy related to COVID-19.41 The angiotensin converting enzyme 2 (ACE2) has been identified as the entry receptor of SARS-CoV-2.42 Both in OSA and in obese patients, there is an increased expression of ACE2 receptors, which would favor the union of the virus.43 In turn, OSA may lead to an increase in blood pressure through stimulation of the renin–angiotensin–aldosterone system.44 Cardiac complications in COVID-19 include myocarditis, ischemic heart disease, heart failure, and arrhythmias,45 factors also associated with OSA, which can increase morbidity and mortality.46
Extra care and close monitoring should be provided to patients with OSA to minimize the risk of infections. Simple questionnaires such as STOP-Bang questionnaire can be used for screening patients who may be at risk for severe adverse outcomes.
Finally, changes in the adherence to continuous positive airway pressure machine (CPAP) treatment have been seen during the pandemic period. New technologies and telemedicine should help us to improve the efficacy and control of treated patients and the adherence to treatment.
Lung cancerSince the World Health Organization (WHO) declared the SARS-CoV-2 pandemic (COVID-19), the care of lung cancer (LC) patients has been particularly compromised. The International Agency for Research on Cancer (ARC) estimated more than 2 million new cases of LC by 2020,47 and some countries have changed the trend in the LC mortality.48 However, these estimates are made without including the effects of the pandemic, so the number of diagnosed cases is likely to be lower.49
This is due to several reasons; firstly, because LC screening programs (which have demonstrated several beneficials in the context of the high prevalence of LC)50–53 are interrupted due to the existing health care bottleneck and to avoid transmission of infection to both patients and health care workers.54,55 This delay in screening may not have an impact on survival as long as it does not extend beyond 18 months.56,57 On the other hand, the number of consultations decreases either because users do not come to the hospital because of fear of contracting the disease, or because they are not referred to the hospital due to both the health care overlap and the overlap of symptoms between COVID-19 and LC.58,59
With the advent of the pandemic, the ARC's projected LC mortality of 18% by 2020 is also likely to change.47 Patients with LC are more susceptible to contracting COVID-1960,61 and to developing complications from the disease. Rogado et al.62 estimate an incidence of 0.9% compared to 0.5% in the general population. Because of this, higher hospitalization and mortality rates have also been observed,63,64 which in the study by Rogado et al.62 was 52.3% in patients with LC compared to 10.2% in the general population. Retrospective studies have reported a rate of COVID-19 infection and serious complications up to 2.31 times higher than the general population or patients with other cancers.65–67 In an Italian study, a 4-fold increased risk of dying from COVID-19 infection was observed. Specifically, 2/3 of patients with LC required hospitalization and a quarter of them died.68 In the TERAVOLT study, the mortality rate was 33%, and the risk of death was associated with older age, presence of comorbidities and active smoking,64 although in a recent meta-analysis,69 where mortality was higher in COVID-19 patients (RR, 1.66 vs 1.33) as well as the risk of ICU admission (RR, 1.56 vs 1.31), no increased risk of death or serious complications was found in relation to advanced age.
Fear of contracting the disease has also led to a significant delay in the diagnosis of these patients. In a study on the participation of cancer patients in social networks during the pandemic, the highest level of participation was among LC patients (38.3%) followed by breast cancer patients (23.8%). The most predominant emotion related to COVID-19 and cancer was fear,70 and is primarily linked to the fear of contracting the disease and not being admitted to the ICU if necessary.71,72 In a study by Garassino et al.,64 75% of patients with LC and COVID-19 were admitted to hospitals, but only 10% were admitted to the ICU. Thus, the delay in diagnosis has led to an increase in the diagnosis of more advanced cases. A retrospective study analyzed 161 LC patients of whom 29.19% had a delay in their scheduled appointment, compared to 5% of delays before the pandemic. In addition, 18.18% of the delayed patients had advanced disease.65 A study carried out in England73 estimates that delay in diagnosis leads to an increase in years of life lost and an increase in mortality of 4.8% at 5 years after diagnosis.
Treatment with chemotherapy in the months prior to diagnosis of COVID-19 has also been associated with an increased risk of serious infection with complications (hazard ratio [HR] 1.71).64,66,74,75 A recent meta-analysis76 of 3558 patients shows an increased risk of death in patients with active chemotherapy compared to those without (OR 1.60). Although there may be confusion between symptoms caused by COVID-19 and chemotherapy-related chemical pneumonitis, it is important to assess the risk-benefit of discontinuing treatment77 as the use of immunotherapy improves the prognosis of patients with few side effects, so it is recommended to prioritize its use over conventional chemotherapy.60,78
Not only the delay in treatment but also the suspension of consultations, surgery and treatments such as chemotherapy or immunotherapy79 have had an impact on patients with LC, as they have led to disease progression and increased mortality from LC.54 In a study of 288 hospitalised patients with COVID-19 and LC, disease progression was estimated in 10.7% of cases due to discontinuation of radiotherapy and 26% due to discontinuation of chemotherapy and/or immunotherapy.80 Regarding surgery, in the first publication of the CovidSurg Cohort Study, a mortality of 42.9% was observed in resection surgery.81 However, in a subsequent multicentre, prospective study involving 140,231 patients, the same authors observed that the 30-day mortality rate in resection surgery was higher at 2, 4 and 6 weeks after diagnosis of COVID-19 (OR 4.1, 3.9 and 3.6, respectively), but was similar at 7 weeks (OR 1.5), except in those who still had symptoms, where mortality was still higher than if they were asymptomatic (6% vs. 1.3%).82 Therefore, some authors recommend stereotactic body radiation therapy as a first curative option if surgery has to be delayed due to lack of operating theaters.83 Therefore, the treatment strategy must be adapted to different factors, such as the incidence of COVID-19 or the risk of delaying surgery beyond 3 weeks. In this regard, the American Society for Thoracic Surgery makes recommendations that cover all these aspects.84
The impact on the family and social support usually provided to these patients has had a negative impact on their quality of life. Depression in cancer patients, especially in women, has increased during the pandemic.65
A balance needs to be struck between healthcare for COVID-19 patients and care for LC patients. The role of telemedicine is crucial to avoid unnecessary hospital visits,85 but without losing the human aspect of medicine, which in these LC patients is as important as traditional oncology treatment.86
BronchiectasisRadiological detection of bronchiectasis is frequent in the acute phase of COVID-19, and may even represent a factor of its severity.87–89 In addition, there are frequent sequelae in patients who have overcome the infection,90,91 although their long-term clinical repercussion is unknown.92 The literature on the incidence or severity of COVID-19 in patients with pre-existing bronchiectasis is scarce, probably due to the low proportion of these patients in the published series on COVID-19 patients.93,94
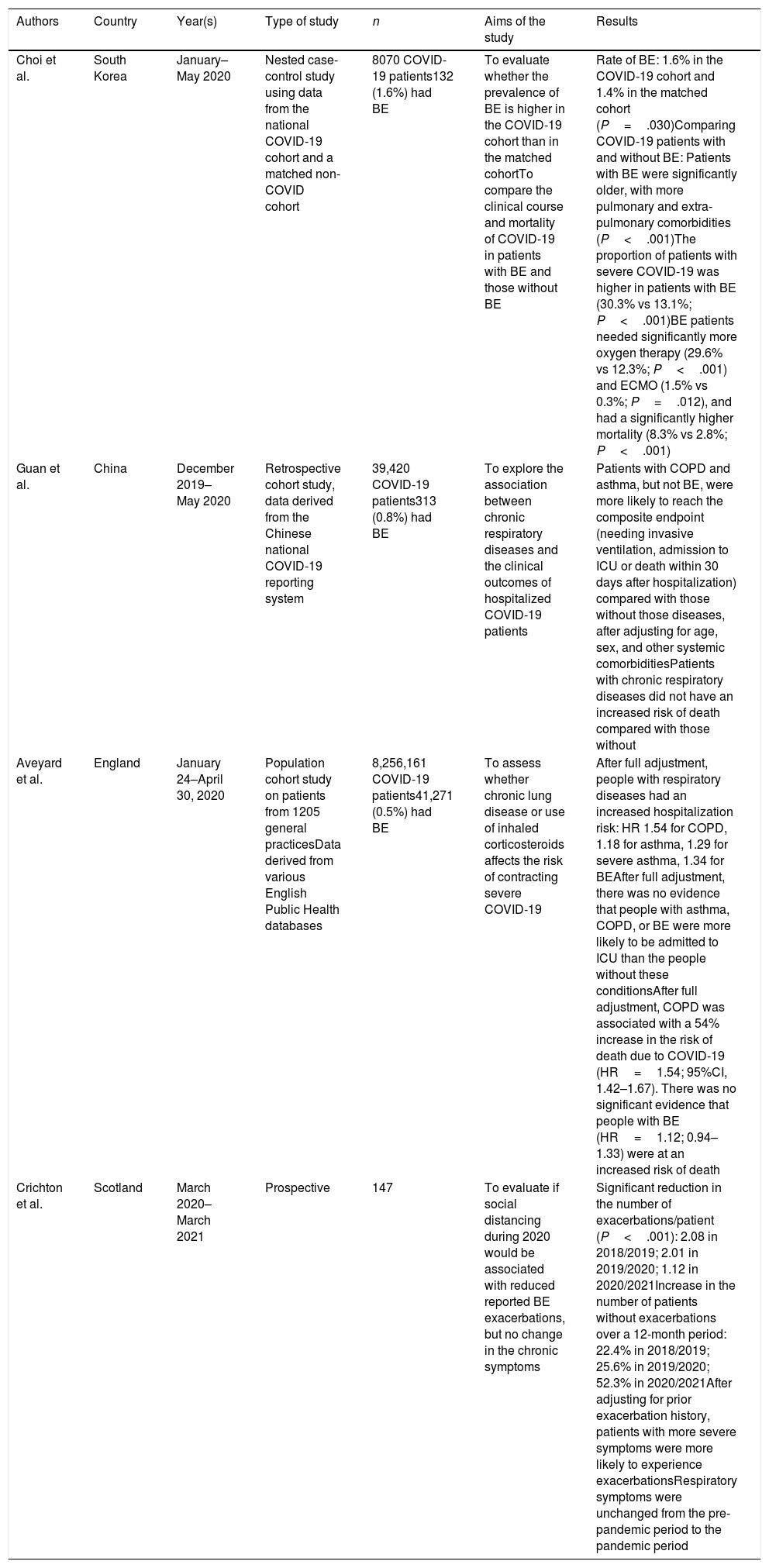
Table 1 lists the main publications that have in some way addressed the relationship between bronchiectasis and COVID-19, of which only two were specifically designed to answer clinical questions in this population.95–98 Although the results of these studies are very disparate, and even contradictory, it could be highlighted that when comparing COVID-19 patients with and without bronchiectasis, the former have an increased risk of developing severe forms of the infection, requiring oxygen therapy or hospitalization, and possibly also an increased risk of requiring admission to an intensive care unit or even death. These series lack relevant data that could allow a more in-depth interpretation of these findings, such as severity or chronic bronchial colonization.
Publications that have examined the relationship between bronchiectasis (BE) and COVID-19.
| Authors | Country | Year(s) | Type of study | n | Aims of the study | Results |
|---|---|---|---|---|---|---|
| Choi et al. | South Korea | January–May 2020 | Nested case-control study using data from the national COVID-19 cohort and a matched non-COVID cohort | 8070 COVID-19 patients132 (1.6%) had BE | To evaluate whether the prevalence of BE is higher in the COVID-19 cohort than in the matched cohortTo compare the clinical course and mortality of COVID-19 in patients with BE and those without BE | Rate of BE: 1.6% in the COVID-19 cohort and 1.4% in the matched cohort (P=.030)Comparing COVID-19 patients with and without BE: Patients with BE were significantly older, with more pulmonary and extra-pulmonary comorbidities (P<.001)The proportion of patients with severe COVID-19 was higher in patients with BE (30.3% vs 13.1%; P<.001)BE patients needed significantly more oxygen therapy (29.6% vs 12.3%; P<.001) and ECMO (1.5% vs 0.3%; P=.012), and had a significantly higher mortality (8.3% vs 2.8%; P<.001) |
| Guan et al. | China | December 2019–May 2020 | Retrospective cohort study, data derived from the Chinese national COVID-19 reporting system | 39,420 COVID-19 patients313 (0.8%) had BE | To explore the association between chronic respiratory diseases and the clinical outcomes of hospitalized COVID-19 patients | Patients with COPD and asthma, but not BE, were more likely to reach the composite endpoint (needing invasive ventilation, admission to ICU or death within 30 days after hospitalization) compared with those without those diseases, after adjusting for age, sex, and other systemic comorbiditiesPatients with chronic respiratory diseases did not have an increased risk of death compared with those without |
| Aveyard et al. | England | January 24–April 30, 2020 | Population cohort study on patients from 1205 general practicesData derived from various English Public Health databases | 8,256,161 COVID-19 patients41,271 (0.5%) had BE | To assess whether chronic lung disease or use of inhaled corticosteroids affects the risk of contracting severe COVID-19 | After full adjustment, people with respiratory diseases had an increased hospitalization risk: HR 1.54 for COPD, 1.18 for asthma, 1.29 for severe asthma, 1.34 for BEAfter full adjustment, there was no evidence that people with asthma, COPD, or BE were more likely to be admitted to ICU than the people without these conditionsAfter full adjustment, COPD was associated with a 54% increase in the risk of death due to COVID-19 (HR=1.54; 95%CI, 1.42–1.67). There was no significant evidence that people with BE (HR=1.12; 0.94–1.33) were at an increased risk of death |
| Crichton et al. | Scotland | March 2020–March 2021 | Prospective | 147 | To evaluate if social distancing during 2020 would be associated with reduced reported BE exacerbations, but no change in the chronic symptoms | Significant reduction in the number of exacerbations/patient (P<.001): 2.08 in 2018/2019; 2.01 in 2019/2020; 1.12 in 2020/2021Increase in the number of patients without exacerbations over a 12-month period: 22.4% in 2018/2019; 25.6% in 2019/2020; 52.3% in 2020/2021After adjusting for prior exacerbation history, patients with more severe symptoms were more likely to experience exacerbationsRespiratory symptoms were unchanged from the pre-pandemic period to the pandemic period |
On the other hand, as in other chronic respiratory diseases, a notable decrease (nearly 50%) in exacerbations of bronchiectasis has been observed.95 Given the important prevalence of both bronchiectasis and chronic bronchial infection,99,100 this supposes a great epidemiological impact. This is probably due to a reduction in viral infections motivated by social distancing measures, which this population has taken to the extreme, both due to the fear of suffering severe COVID-19, and the stigmatization that chronic cough entails in many of these patients.101 Finally, there are publications on patients with bronchiectasis generating diseases, such as common variable immunodeficiency102 or ciliary dyskinesia,103 which show a low incidence of COVID-19. In these diseases, the severity of COVID-19 is associated with the concomitant presence of respiratory diseases, such as bronchiectasis or interstitial lung disease.
Cystic fibrosisThe impact of SARS-CoV-2 infection in patients with cystic fibrosis (CF) was lower than expected,104–107 but older patients, those with lower lung function, CF-related diabetes and those who received a solid organ transplant108–110 experienced a more unfavorable evolution.
The cumulative incidence was variable across the different series analyzed, between 2.7/1000 CF patients,110 3.2/1000 CF patients,111 and 17.2/1000 CF patients.112 Both short and long-term effects of the pandemic were observed in patients with CF (Table 2).
Short and long-term changes in cystic fibrosis patients in relation to the COVID-19 pandemic.
| Major and rapid changes were necessary for the health care system |
| The lower number of in-person visitsLower risk of cross-infection |
| Preventive measures such as using face masks and hand hygiene were reinforced |
| Decrease in the exacerbations number (absence of other viral infection) |
| Patients reduced their physical activity during lockdown periods |
| Increased anxiety and depression symptoms |
| Decrease in the number of lung function tests made at the hospitalSpirometry and oxygen saturation date were recorded at home |
| Sputum for microbiological cultures was recorded at home |
| Fewer lung transplantation was performed |
| Reduced clinical trials: Multiple challenges for recruiting and following patients |
| It was necessary to implement telehealth and other monitoring systems for the future |
| It will be a challenge how to achieve appropriate levels of immunization in CF patients with a lung transplant |
| The long-term effects of COVID-19 on the CF population remain unknown |
During the COVID-19 pandemic, there was a significant decrease in exacerbations and hospitalizations,113 likely as a result of the use of barrier measures and the reduction of other viral infections. Nevertheless, rapid changes were required in the healthcare systems114 to prevent these patients from being exposed to the virus. Also, for this reason patients reduced their physical activity,115,116 with the resulting muscular damage and increased anxiety-depression symptoms117–119 that will lead to a need for future medical attention.
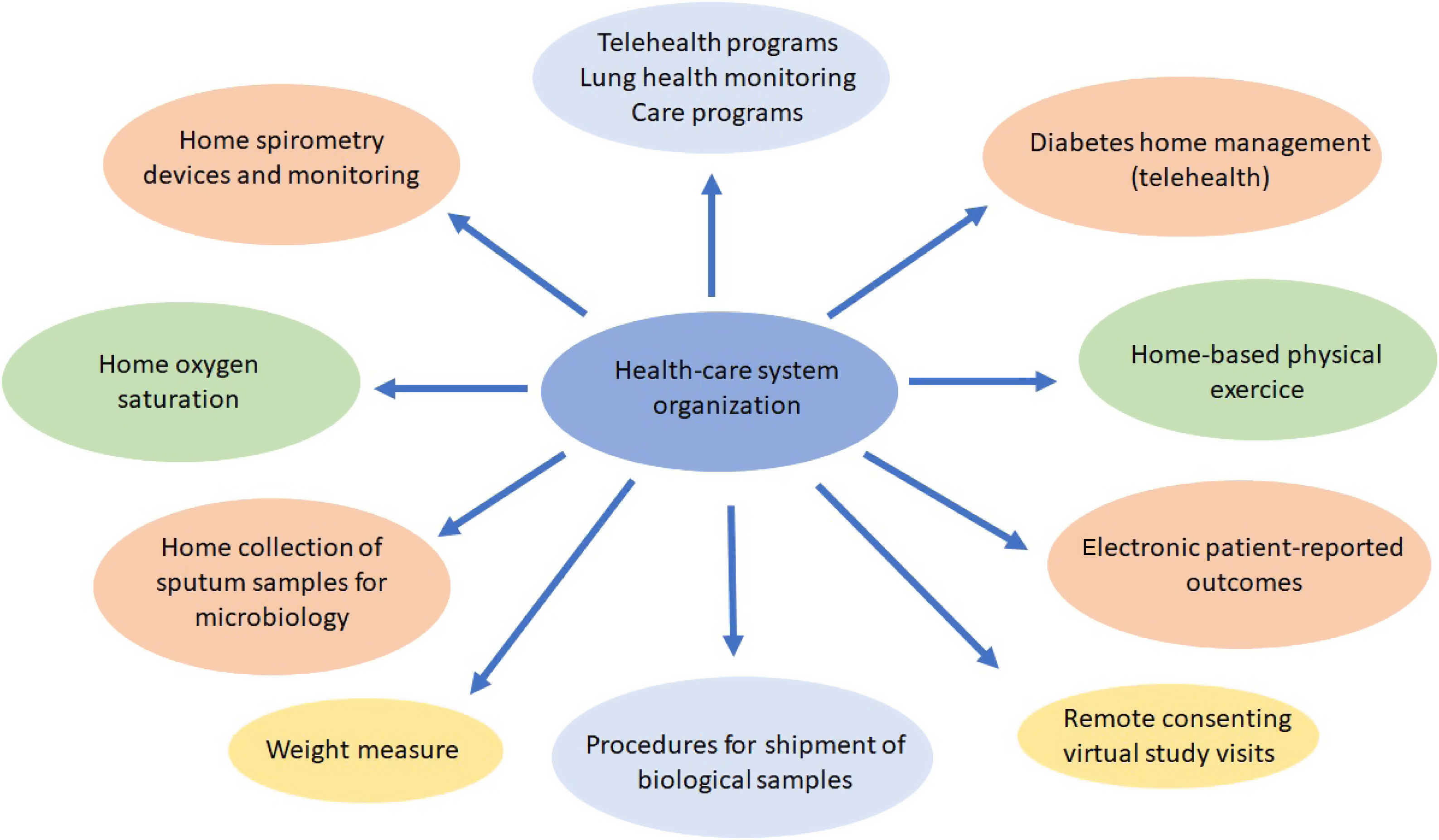
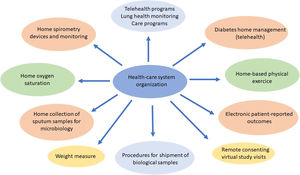
Procedures such as respiratory function tests have been drastically reduced and remote attention such as telehealth,120–123 home spirometry,108 home collection of sputum samples,124 home delivery of medication from the hospital pharmacy, and long-distance electronic monitoring123 were required. This provided an opportunity to take new approaches to improving CF patients care with new and effective models, with durable changes in the future CF health care (Fig. 1).
It is also important to highlight that fewer transplant125,126 were performed although, in CF patients, the use of triple modulatory therapy just at the beginning of the pandemic was able to minimize the impact and severity of these patients.123,127
Finally, clinical trials were significantly reduced due to the difficulties in the recruitment and the follow-up visits to patients, having a negative impact on future treatment options.108,123,128
At that moment, the long-term effects of COVID-19 on the CF population remain unknown. In the near future, possible cardiovascular symptoms, cognitive deficit and fatigue may appear in CF patients who have passed the infection. Another important issue concerns vaccination for CF transplant patients.108,129 Maybe, it should be explored to use extra doses or using different combination vaccines to achieve appropriate levels of immunization.
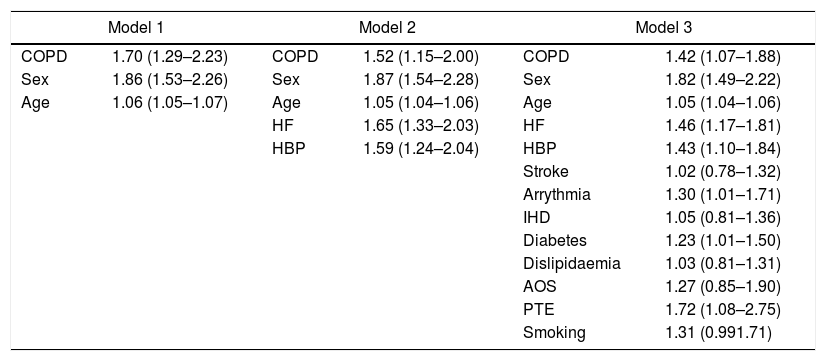
Chronic obstructive pulmonary diseaseSince the description of the first cases of SARS-CoV-2 infection, advanced age and several chronic diseases have been associated with a higher risk of unfavorable evolution.130–132 In our setting, a higher incidence of COVID-19 has been described in chronic obstructive pulmonary disease (COPD) patients (2.51%; 95%CI, 2.33–2.68) compared to the general population over 40 years of age (1.16%; 95%CI, 1.14–1.18; P<.001), with a worse prognosis, assessed by the number of hospitalizations and higher mortality.133 Patients with COPD and COVID-19 were predominantly male, smoked more frequently, and had more comorbidities than patients without COPD.134 Using various adjustment models, COPD itself posed a higher risk of COVID-19, regardless of age and the associated comorbidities that these patients frequently present135 (Table 3). Before vaccines were available, most COPD patients admitted for COVID-19 had pulmonary infiltrates compatible with pneumonia, causing a higher rate of hospitalizations and in-hospital mortality. This evolution contrasts with the findings observed in patients with COPD exacerbations due to other viral etiologies.133
OR (95%CI) for risk of death in COVID-19 patients with COPD versus those without COPD, adjusted for most relevant covariates, in three models of multivariate logistic regression analysis.133
| Model 1 | Model 2 | Model 3 | |||
|---|---|---|---|---|---|
| COPD | 1.70 (1.29–2.23) | COPD | 1.52 (1.15–2.00) | COPD | 1.42 (1.07–1.88) |
| Sex | 1.86 (1.53–2.26) | Sex | 1.87 (1.54–2.28) | Sex | 1.82 (1.49–2.22) |
| Age | 1.06 (1.05–1.07) | Age | 1.05 (1.04–1.06) | Age | 1.05 (1.04–1.06) |
| HF | 1.65 (1.33–2.03) | HF | 1.46 (1.17–1.81) | ||
| HBP | 1.59 (1.24–2.04) | HBP | 1.43 (1.10–1.84) | ||
| Stroke | 1.02 (0.78–1.32) | ||||
| Arrythmia | 1.30 (1.01–1.71) | ||||
| IHD | 1.05 (0.81–1.36) | ||||
| Diabetes | 1.23 (1.01–1.50) | ||||
| Dislipidaemia | 1.03 (0.81–1.31) | ||||
| AOS | 1.27 (0.85–1.90) | ||||
| PTE | 1.72 (1.08–2.75) | ||||
| Smoking | 1.31 (0.991.71) | ||||
95%CI: 95% confidence interval; COPD: chronic obstructive pulmonary disease; HBP: high blood pressure; HF: heart failure; IHD: ischemic heart disease; OR: odds ratio; OSA: obstructive sleep apnea; PTE: pulmonary thromboembolism.
At present, there is no data that the treatment of COPD or its comorbidities has a negative impact on prognosis.136 Although a study described that the use of inhaled corticosteroids in COPD increased the risk of developing COVID-19, this result is conditioned by an indication bias due to severity.137 For this reason, patients should continue with their usual treatment without changes, avoiding the use of nebulizers as much as possible, especially if they do not have a filter in the expiratory branch.138
Given the increased risk that COPD patients have, it is recommended that they strictly follow self-care measures that help prevent SARS-CoV-2 infection, including the use of a face mask.139 There is currently no data indicating that the effect of the vaccine is different in COPD patients than in the general population.
Vascular pulmonary diseasesThe ability of SARS-CoV-2 to invade vascular endothelial cells leads to endothelial inflammation, thrombin generation, and platelet and leukocyte recruitment causing a predisposition to thrombotic phenomena in different sites.140–142 Thromboembolism rates of COVID-19 were high and associated with higher risk of death in hospitalized patients.143–145 Pulmonary embolism (PE) prediction in COVID-19 patients is challenging. Some factors independently associated with PE were tachypnea, absence of infiltrates in the chest X-ray and elevated D-dimer levels,146 which per se are already high in patients with a worse prognosis.147
Anticoagulation for prophylactic purposes is recommended in COVID-19 patients. In addition, low molecular weight heparin (LMWH) has also been suggested to have anti-inflammatory and antiviral properties.148 Up to now, findings provide comprehensive and high-quality evidence for the use of standard-dose prophylactic anticoagulation over an escalated-dose regimen as routine standard of care for hospitalized COVID-19 patients irrespective of disease severity.149 However, a new paradigm has been opened and some clinical guidelines150 consider a treatment dose of LMWH for COVID-19 patients who need low-flow oxygen and who do not have an increased bleeding risk.
Treatment patterns have also been modified during the pandemic because there has been an increase in the use of direct oral anticoagulants (DOACs) and prolongation of days with LMWH. Although the long-term outcomes in survivors of COVID-19 are unknown at present, thrombotic sequelae have the potential to become a clinically significant problem.151 Routine post-COVID-19 follow-up pathways should include lung perfusion imaging and cardiopulmonary exercise test.152,153
The reasons of an unexpected favorable incidence of COVID-19 in patients with pulmonary hypertension (PH) and chronic thromboembolic pulmonary hypertension (CTEPH) are not yet clarified.154
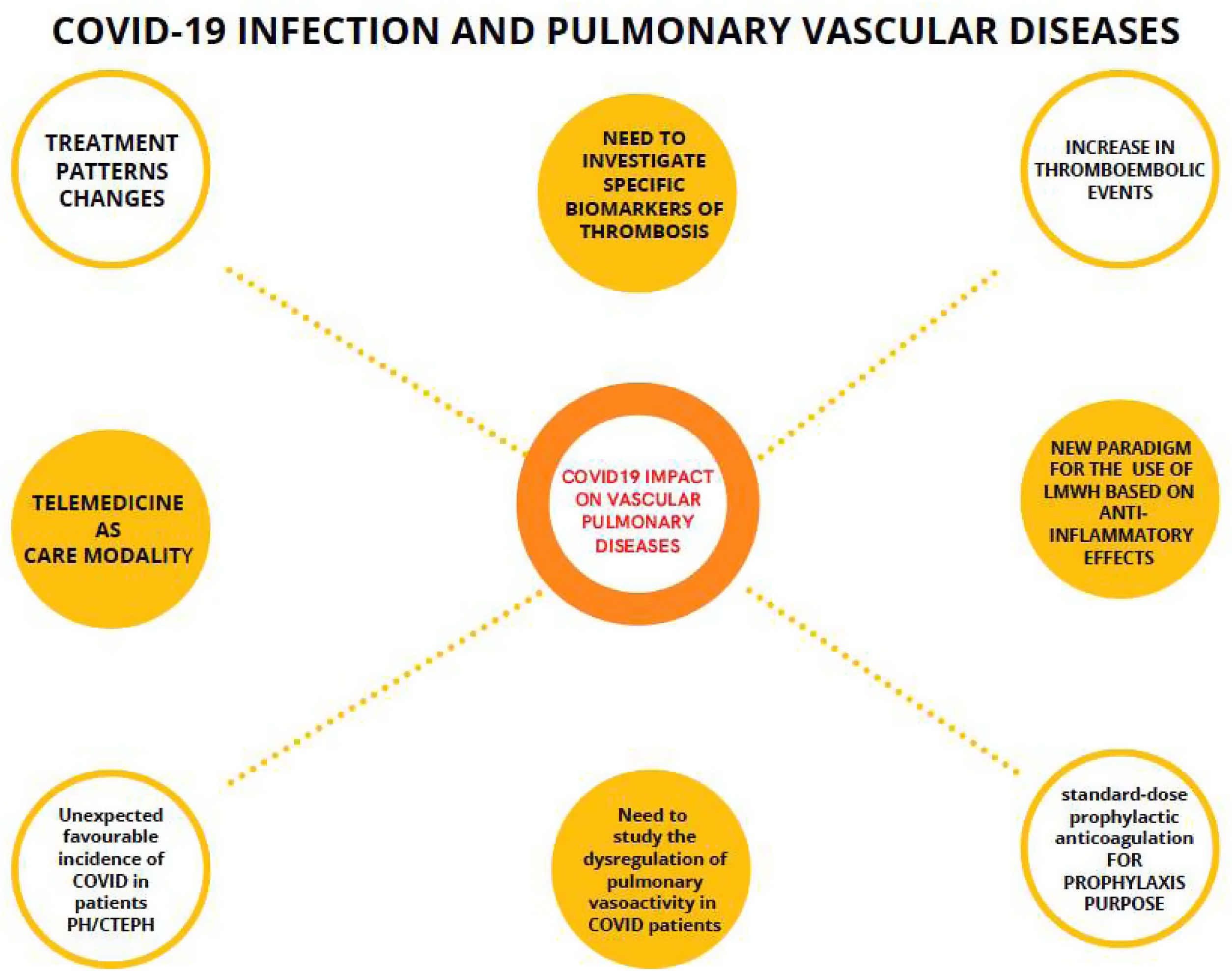
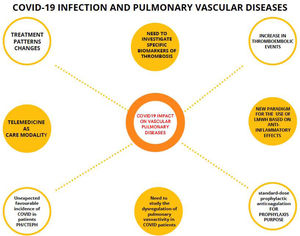
The usefulness of telemedicine during the pandemic in all medical specialties has been demonstrated, both in acute episodes and after hospital discharges155 (Fig. 2).
Mind map of the impact of COVID-19 on pulmonary vascular diseases. Connected by dashed lines, the evidences in relation to COVID-19 and pulmonary vascular diseases. Unconnected circles are thoughts of the COVID-19 pandemic. LMWH: low molecular weight heparin; PH/CTEPH: pulmonary hypertension and chronic thromboembolic pulmonary hypertension.
Chronic inflammation (typically T2) and bronchial remodeling, usual features of asthma, could increase the susceptibility to COVID-19. Besides, it has been shown that a T1 predominant inflammatory pattern—and not T2—is associated with higher expression of ACE2 receptor.156 It has been controversial whether asthma augments the risk of becoming infected but, in the light of the recently published studies, it might be argued that the risk is restricted to severe forms of the disease.97,137,157–162 The EAVE II study showed that adults with asthma had an increased risk of COVID-19 hospital admission (adjusted HR 1.27; 95%CI, 1.23–1.32) compared with those without asthma, and that those who have required ≥2 oral corticosteroids bursts in the previous 2 years are at increased risk of ICU admission or death, even accounting for vaccination status.163 In children, a recent meta-analysis concluded that asthma does not appear to be a risk factor for hospitalization or ICU admission caused by COVID-19.164 On the other hand, unscheduled asthma visits appeared to be significantly reduced during COVID-19 pandemic,165 likely owing to reduced viral upper respiratory tract infections.
Inhaled corticosteroids (ICS) might potentially favor viral replication and delay viral clearance but, on the contrary, they might reduce the epithelial expression of ACE2 and TMPRSS2 (viral entry receptors)166 and also the levels of potentially harmful cytokines such as IL-6 and IL-8.167,168 Investigations on the therapeutic effect of ICS in COVID-19 patients yielded controversial—although mostly favorable—results.169–173 A meta-analysis supports ICS use to short the duration of the disease and, maybe, to prevent hospitalizations.174
Biologics can negatively impact B lymphocytes differentiation into plasma cells and the generation of plasma memory cells through eosinophil depletion,175 and, in fact, humoral vaccine response is lower in patients who are receiving this treatment.176 By contrast, omalizumab has been shown to restore the capacity of plasmacytoid dendritic cells to produce alpha interferon (IFN-α), promoting their antiviral activity.177 According to results from large databases and several national registries, biologic therapy does not increase risk for severe COVID-19 in asthma patients.178–180
Interstitial lung diseasesThe pandemic of COVID-19 has significantly impact on patients with interstitial lung diseases (ILDs). In general, patients with pre-existing respiratory disease, including interstitial lung diseases, have an increased risk of severe SARS-CoV-2 infection97,181 and increased risk of mortality.182 Recent publications have focused specifically on the impact of COVID-19 infection in ILDs.183–186 A recent national Korean study compared two cohorts of patients with and without COVID-19, observing that the proportion of patients with ILDs was significantly higher in the COVID-19 cohort than in the matched cohort (adjusted OR 2.02; 95%CI, 1.54–2.61). Mortality is also higher in patients with ILDs compared to general population.183–186 In addition, patients with fibrotic ILDs have probably higher rates of hospitalization and mortality compared to other ILDs.183,185
After severe COVID-19 infection, pulmonary sequalae could be a long-term complication, including lung fibrosis.187,188 The consequences after COVID-19 infection in patients with ILDs are not clear. The possibility of acute exacerbation after COVID-19 infection has been described.189 Viral infections could be the trigger of lung fibrosis exacerbation/progression. The potential fibrotic effect of SARS-CoV-2 infection in a fibrotic environment could have a huge impact in the prognosis of ILDs.190 The real long-term impact of COVID-19 infection in ILD patients must be elucidated in the future.
General prevention measures (masks, social distance) and vaccination have been recommended to avoid risk of infection. Surprisingly, some cases of acute exacerbation after COVID-19 vaccination have been described in ILDs.191–193 The benefit of vaccination is over the potential side effects of the vaccine, but physicians must monitor closely those patients that present respiratory worsening after vaccination.
As patients with ILDs are a fragile population in the context of COVID-19 pandemic, the management of these diseases has been consequently affected. Major issues for patients with ILDs have been described: restricted access to the diagnostic process, uncertainties in the use of common ILDs pharmacotherapies, limited ability to monitor both disease severity and medication adverse effects, and significantly curtailed research activities.194 The development of telematic technologies rapidly raised during this period. Telehealth and home monitoring could be an option in the future for ILDs,195,196 but it still needs more development to be fully included in the habitual clinical management.
SmokingGiven the statement that some authors have made as to whether nicotine could be a therapeutic option, even protective, for COVID-19 infection,197 Takagi198 performed a meta-regression in which he demonstrated a positive association between the smoking prevalence and COVID-19 infection, independent of other co-variables; therefore, the hypothesis that the prognosis of the disease is better for being a smoker is not supported. Conversely, in this moment it is no doubt that current and past smoking produces a more severe clinical form of COVID-19 and more frequently leads these patients to be admitted to ICU, are intubated, and die.199,200 This association was more significant for former smokers than in current smokers,201 although some studies have found an evident trend toward a worse progression in smokers.200 Clearly, smoking is an independent risk for having progression of COVID-19, including mortality,202 and people who smoke are at an increased risk of developing symptomatic COVID-19.203
Findings in the same line have been found between the consumption of electronic cigarettes (EC) and the infection by COVID-19: Vapers experience higher frequency of COVID-19 related symptoms when compared with age and gender matched non-vapers.204
Another question is, has the COVID-19 infections impacted smokers? A United Kingdom study205 in adults investigated changes in cigarette and EC use during the COVID-19 pandemic and what factors were associated with the changes. They found that many smokers and vapers tried to quit and a high proportion succeeded, others cut down or stayed the same. The pandemic provided motivation to quit smoking and on the other hand prompted vapers to return to cigarette smoking.205,206 In Pakistan207 like in Jordan,208 while many people stopped, reduced, or tried quitting smoking, some increased smoking, and some relapsed after quitting. Smokers in China209 on average reduced their tobacco consumption after the nationwide viral outbreak had been contained, but there were differences: men, those with a longer history of consumption or residing in urban areas were less able to reduce their consumption. Some authors found that a majority of smokers were not affected by the pandemic in their consumption even knowing the effect of this association,210,211 and it has even been reported that many smokers have smoked more to calm down and deal with negative feelings and social impacts.212 It is recognized that in the pandemic the motivation to quit smoking has increased slightly, correlating the above with social and well-being changes, but there were no changes in final abstinence or in the willingness to quit cigarettes, although the tendency to try an electronic cigarette increased.213,214
ConclusionsCOVID-19 can affect the respiratory system in a variety of ways and across a spectrum of levels of disease severity, depending on a person's immune system, age and comorbidities. It is important that patients who have underlying lung disease can certainly have worsening of those conditions with contraction or exposure to COVID-19. Global public health effort is required to increase awareness about minimizing the burden of these comorbidities conditions that cause fatalities in COVID-19 infected peoples.
Conflict of interestsThe authors declare that they have no conflict of interest.