Malnutrition and sarcopenia are prevalent and often underdiagnosed in individuals with chronic obstructive pulmonary disease (COPD), with significant implications for morbidity, mortality, and quality of life. Despite their clinical relevance, current COPD guidelines provide limited direction regarding nutritional assessment and intervention. This consensus document, developed jointly by the Spanish Society of Pulmonology and Thoracic Surgery (SEPAR) and the Spanish Society of Endocrinology and Nutrition (SEEN), offers evidence-based recommendations for the nutritional assessment and management of patients with COPD. A multidisciplinary panel of experts identified three key clinical issues relating to the prevalence and impact of malnutrition, appropriate assessment strategies, and the effectiveness of nutritional interventions. Systematic reviews were conducted in accordance with PRISMA guidelines, and the quality of evidence was assessed using SIGN and GRADE methodologies. These recommendations aim to support clinical decision-making and promote early, structured, and individualized nutritional strategies to improve outcomes in patients with COPD.
Chronic obstructive pulmonary disease (COPD) is one of the chronic conditions that consumes the most healthcare and economic resources, representing a major public health issue. Today, this disease is recognized as a heterogeneous entity in which, in addition to pulmonary involvement, systemic manifestations may also be present. Among these, malnutrition stands out. The relationship between the two conditions is complex and very likely bidirectional. Despite their connection and the impact that may result from the coexistence of both conditions, nutritional assessment in the care of patients with COPD remains inadequate. This may be partly due to the limited attention that COPD management guidelines give to this non-pharmacological intervention, which has received little focus—except for the recently published GARIN guideline [1]. All of this justifies the development of the present consensus document, whose main objective is to comprehensively address the nutritional assessment and management of patients with COPD. Its creation is the result of collaboration between experts from the Spanish Society of Pulmonology and Thoracic Surgery (SEPAR) and the Spanish Society of Endocrinology and Nutrition (SEEN).
MethodologyTwo different evidence assessment methodologies were used in the development of this document. The first two questions were addressed using the Scottish Intercollegiate Guidelines Network (SIGN) methodology, while the third question was approached using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) methodology.
The process began with the formation of an expert panel composed of members from SEPAR and SEEN. This multidisciplinary group reached a consensus on three key questions that guided the development of the document. A systematic literature review was then conducted following the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) methodology. Each phase of the review was carried out in duplicate, and in cases of disagreement, a third reviewer was involved to resolve the discrepancies.
For the final phase, the panel was divided into three subgroups according to the clinical specialty of its members, allowing for a more specific and detailed analysis of the evidence. As a result of this collaborative effort, the recommendations were developed. Finally, the document was reviewed and approved by all the authors (Supplementary Materials 1–4).
QuestionsWhat is the prevalence and impact of malnutrition on the clinical and functional progression of patients with COPD?PrevalenceThe prevalence of malnutrition in COPD is not well established. Prior to the publication of the Global Leadership Initiative on Malnutrition (GLIM) diagnostic criteria (Supplementary 1 Table 1) [2]—developed with the participation of leading clinical nutrition societies from most continents—there was no consensus on how to diagnose malnutrition. As a result, the tools used to identify it across different studies have not been uniform. This variability in criteria, combined with country-specific socioeconomic factors, study settings, and the clinical status of patients at the time of assessment (whether in a stable phase or during an exacerbation), leads to highly variable prevalence rates [3–7]. A recent meta-analysis estimated the global prevalence of malnutrition in COPD patients at 30%, with wide regional variations ranging from 16.1% to 53.5% [5]. In recent years, the prevalence of malnutrition in individuals with COPD—diagnosed using GLIM criteria—has been estimated to range from approximately 22–48% [8–11].
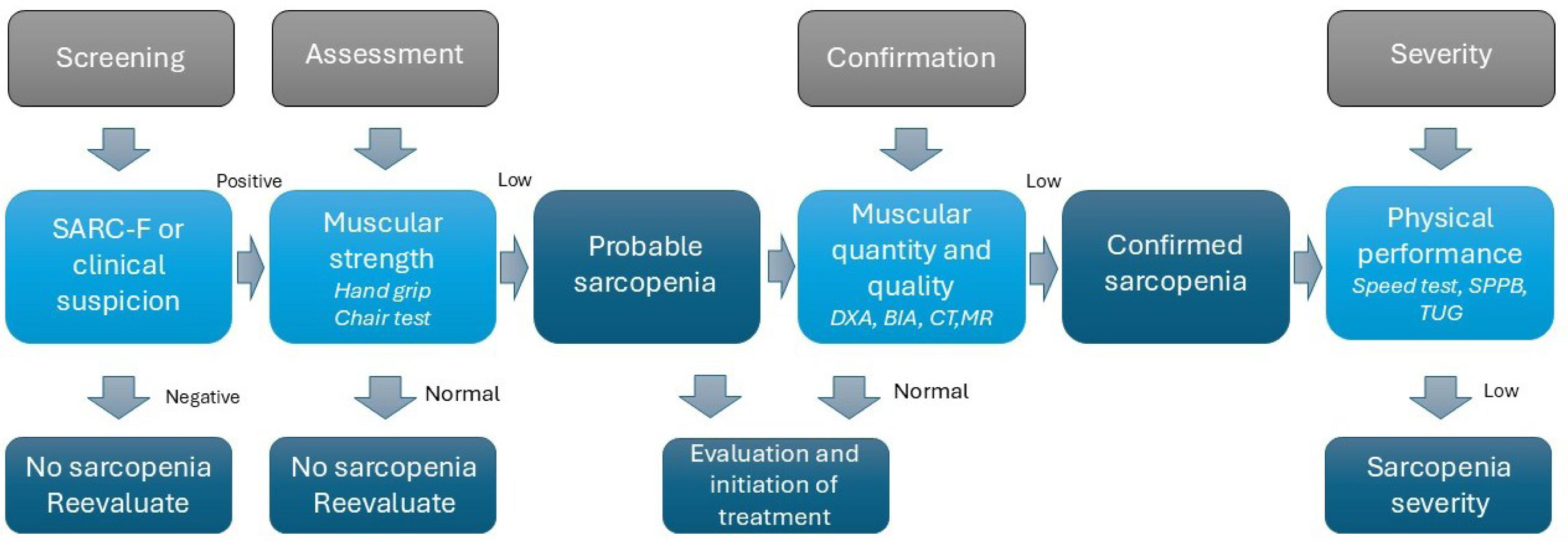
Sarcopenia is defined as a progressive and generalized skeletal muscle disease characterized by the loss of muscle strength, mass, and quality, leading to unfavorable clinical outcomes [12]. Fig. 1 shows the diagnostic algorithm for sarcopenia according to the revised criteria of the European Working Group on Sarcopenia in Older People (EWGSOP2). This condition is frequently observed in patients with COPD, with a global prevalence of 21.6% [13], and it can go unnoticed in some patients with a high body mass index (BMI). Additionally, around 25% of people with COPD will develop cachexia (defined as the loss of lean body mass due to chronic disease) during the course of their illness [14]. Although malnutrition, sarcopenia, and cachexia are distinct conditions, they are closely related and often overlap in many individuals, so a proactive approach is necessary to detect them early [9,11].
Diagnosis of sarcopenia according to EWGSOP2 criteria. Abbreviations: BIA: bioelectrical impedance analysis; CT: computed tomography; DXA: dual-energy X-ray absorptiometry; MRI: magnetic resonance imaging; SARC-F: tool proposed to identify individuals at risk of sarcopenia (see Supplementary 2 Table 4); SPPB: Short Physical Performance Battery; TUG: timed up and go test.
A major limitation of the literature review on the clinical impact of malnutrition is that most studies were conducted without considering the current diagnostic criteria for malnutrition or the importance of functionality. Many of these studies use only BMI as a marker of nutritional status and do not take into account morphofunctional assessment—which is a more recent development but may be highly relevant in this type of patient.
Pulmonary functionSeveral studies have analyzed the association between impaired nutritional parameters and greater severity of COPD. Most of these studies, generally cross-sectional in design, have evaluated pulmonary function variables. Due to the study methodologies, only a statistical association can be established, without inferring causality, between malnutrition and poorer pulmonary function. Supplementary 1 Table 2 shows the main studies, most of which confirm an association between deterioration in nutritional indices and worse pulmonary function in COPD [4,15–37].
Nutritional assessment is predominantly performed using anthropometric parameters, especially BMI [4,15–17,19,22,24–26,29–33,35–39], or body composition measurements. The most studied body composition variable is the fat-free mass index (FFMI), estimated by bioelectrical impedance analysis (BIA) [4,15,18,20,22,27,33,37–39]. Few articles employ multidimensional parameters [21,27,34] or biochemical markers [17,23,24]. There are also few studies in which no association or only a marginal association between nutritional parameters and pulmonary function is found [32,34]. It should be noted that two large longitudinal studies found an association between low body weight—defined as a BMI<18.5kg/m2 (among other variables)—and greater decline in pulmonary function over time [35,36]. Data from the multinational ECLIPSE cohort reflect a reduction in nutritional indices such as FFMI and BMI as the severity of airflow obstruction increases [38]. Additionally, a meta-analysis of four clinical trials confirmed that low BMI is a risk factor for significant worsening of pulmonary function, while a BMI>30kg/m2 acts as a protective factor [40]. Other anthropometric parameters, such as reduced calf circumference—which can be considered a surrogate marker of muscle mass—are associated with poorer pulmonary function [41].
Regarding body composition, a decreased FFMI is associated with lower maximal inspiratory pressure (MIP), regardless of whether BMI is normal or low [18,42]. BIA can measure resistance (R), which is the opposition that biological tissue offers to the passage of an electrical current, and reactance (Xc), which is the tissue's ability to store electrical charge like a capacitor. The arctangent of these variables produces the phase angle (PA), a parameter that reflects the integrity of cell membranes and the distribution of intracellular and extracellular water. PA has been shown to be a good prognostic marker in multiple diseases, with lower values indicating worse clinical prognosis. In relation to COPD, PA shows a strong correlation—sometimes even better than FFMI—with various measures of respiratory function such as forced expiratory volume in one second (FEV1) and forced vital capacity (FVC) [39,43,44].
Similarly, the cross-sectional area of the rectus femoris muscle measured by computed tomography (CT) correlates with FEV1 [45]. Additionally, reduced handgrip strength is associated with progressive decline in FEV1 [35]. Several authors have found poorer nutritional parameters, including BMI and FFMI, in patients with CT-detected emphysema compared to other phenotypes, with a correlation also observed between the radiological severity of emphysema and these nutritional markers [20,46].
The influence of categorizing individuals by nutritional risk using the Mini Nutritional Assessment (Supplementary 2 Table 1) on pulmonary function is contradictory, with some studies showing an association with poorer pulmonary function and others showing no such association [21,34].
Level of evidence according to SIGN: 2+. Most of the evidence is based on cross-sectional and some longitudinal studies, which indicate an association between malnutrition—determined by low BMI or reduced muscle mass—and poorer pulmonary function in patients with COPD. However, causality cannot be confidently inferred due to the study designs. Two longitudinal studies suggest an association between a BMI<18.5kg/m2 and a greater decline in pulmonary function over time, which supports the idea that nutritional status clinically impacts the progression of pulmonary function in patients with COPD.
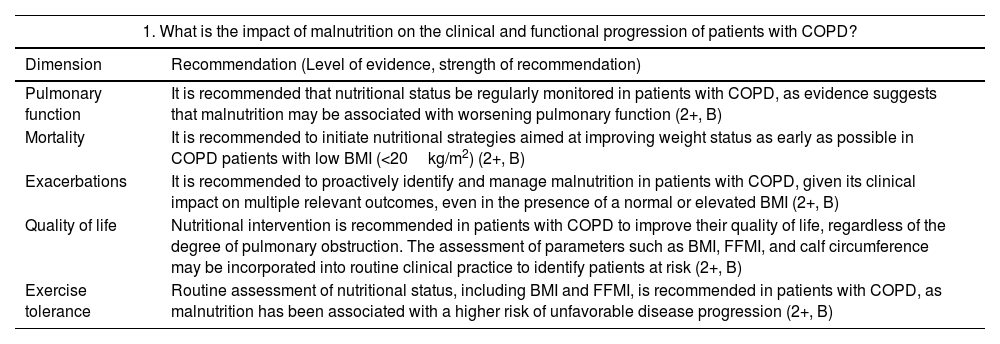
Strength of recommendation: Moderate recommendation (B). It is recommended that nutritional status be regularly monitored in patients with COPD, as evidence suggests that malnutrition may be associated with worsening pulmonary function.
MortalityThe impact of malnutrition on mortality in COPD has been assessed in studies involving outpatients [46,47] or patients hospitalized due to an exacerbation [48–51]. Some of these studies have used administrative databases [50,52], while others are post hoc analyses of clinical trials [53]. Few studies have focused solely on in-hospital mortality [48,50], with most analyzing periods of 2–3 years, and some with longer follow-up durations [54,55]. The vast majority have found an association between poorer nutritional parameters and higher mortality [8,46,48–60]. Supplementary 1 Table 3 presents various studies evaluating the relationship between nutritional parameters and mortality in COPD.
Baseline BMI is the most commonly used parameter [46,48,51–54,56,57]. A low baseline BMI (<18.5kg/m2, <20kg/m2, or <21kg/m2 depending on the authors) has been associated with higher mortality in two meta-analyses [61,62]. In contrast, the influence of overweight or obesity is less consistent. While some studies have found both overweight and obesity to be associated with lower mortality [50,52,61,62], others report that obesity is linked to lower mortality only in patients with advanced-stage COPD [54], or that mild overweight is associated with lower mortality compared to normal weight, but the protective effect disappears at higher degrees of obesity [53].
On the other hand, weight loss over time has been linked to mortality; specifically, involuntary weight loss of more than 5% is associated with a twofold increase in 3-year mortality, showing greater predictive ability than BMI [46,55,63]. However, in some cases, this association may not be consistent [47], possibly because it depends on baseline BMI (in overweight or obese patients, survival appears better in cases of weight loss or weight stability) [55].
Body composition assessment is also related to mortality. Regarding anthropometric parameters, reduced calf circumference (≤34cm in men, ≤33cm in women) is associated with higher two-year mortality in patients with COPD [41]. Mid-arm muscle circumference (MAMC) appears to be a better predictor than BMI. Low values increase the risk of mortality up to fourfold, both in patients with normal weight and those who are overweight [59].
On the other hand, assessment of body composition using advanced techniques is also related to mortality. A reduced FFMI estimated by BIA (defined in most studies as <17kg/m2 or <16kg/m2 in men and <15kg/m2 in women) has been shown to be a prognostic factor for overall and COPD-related mortality, even in individuals with normal weight [58,64–66], as well as its decline over time [66]. Furthermore, a decrease in fat-free mass (FFM) below 80% of the sex-adjusted European reference value has been associated with a fourfold increased risk of mortality [8]. Likewise, raw electrical variables from BIA have demonstrated their importance in this patient profile, with a low phase angle being associated with higher mortality [43,44].
Level of evidence according to SIGN: 2+. Most studies are observational (cohort studies and post hoc analyses) and evaluate the association between malnutrition (measured mainly by BMI, but also by weight loss, FFMI, calf circumference, and phase angle) and increased mortality in patients with COPD, both outpatients and hospitalized. The evidence suggests that low BMI (<18.5–20kg/m2) is correlated with higher mortality in most studies. However, the impact of overweight and obesity is less consistent, with some studies showing a protective effect of mild obesity or overweight under certain circumstances. There is also evidence that weight loss over time is associated with higher mortality, although not all studies agree, possibly due to the influence of baseline BMI. Likewise, there is evidence that reduced muscle mass and phase angle are associated with higher mortality, even independently of BMI.
Strength of recommendation: Moderate recommendation (B). It is recommended to initiate nutritional strategies aimed at improving weight status as early as possible in COPD patients with low BMI (<20kg/m2). There is a strong association between low BMI (<18.5–20kg/m2) and higher mortality in COPD patients, based on the majority of available observational studies. Findings regarding the protective effect of overweight and obesity are not entirely consistent.
Body composition analysis appears to be a better independent predictor of mortality than BMI, whether through anthropometric measures (mid-arm muscle circumference or calf circumference) or advanced techniques (FFMI or phase angle), even in patients with apparently normal weight or overweight.
ExacerbationsMalnutrition impacts other clinical outcomes and is associated with a higher risk of experiencing exacerbations in general, as well as more severe episodes leading to hospital admission (Supplementary 1 Table 4).
There is a high prevalence of underweight, malnutrition, and sarcopenia [7,10,68] in patients with COPD hospitalized due to an exacerbation. Furthermore, these factors are associated with longer hospital stays, a more complicated clinical course, and more frequent subsequent exacerbations [7,24,69,70], which also occurs in those who lose weight afterward [71]. Individuals admitted with malnutrition diagnosed using GLIM criteria and sarcopenia diagnosed by EWGSOP2 criteria have a higher risk of readmission and prolonged hospital stays [11], although this effect disappears in some studies after adjusting for other factors [8]. Overweight and obesity are often accompanied by a low FFMI in patients admitted for an exacerbation, highlighting the importance of the relationship between obesity and sarcopenia [46]. Additionally, malnutrition is associated with a higher number of both early and one-year readmissions following an initial hospitalization [49,50,70,72,73]. Micronutrient deficiencies, especially vitamins B12 and D, are common in patients with acute exacerbations [74]. An albumin level <3g/dL appears to identify a higher risk of prolonged hospitalization [70] and short-term readmission [49,70].
Another recognized impact of malnutrition is a higher risk of hospital admissions in outpatient cohorts, whether defined by GLIM criteria [8] or by the presence of a low BMI [52,58]. Low values of FFM and muscle mass are also associated with an increased risk of readmission, even in the presence of a normal BMI [8,58,70]. Similarly, low BMI values have been linked to a higher risk of exacerbations [19,24,46,47,52,53,58], although in some studies this association loses significance when adjusting for lung function [8] or finds no relationship [46,68,75,76]. Both low BMI and obesity (BMI<22 and >30kg/m2, respectively) are significantly associated with a lower likelihood of presenting a non-exacerbator phenotype in women, with no significant association in men [6]. Likewise, individuals with stable COPD who experience weight loss over time have more exacerbations [47], as do those with reduced calf circumference [41], hypoalbuminemia, or hypoproteinemia [75]. A low score on the Mini Nutritional Assessment Short Form (MNA-SF) screening tool (supplementary 2 Table 1) is also associated with a higher risk of exacerbations [77].
Level of evidence: 2+. Most studies are observational (cohort and cross-sectional studies). There is a consistent association between malnutrition (including low BMI, weight loss, sarcopenia, low FFMI, and hypoalbuminemia) and worse clinical outcomes in patients with COPD, such as a higher risk of exacerbations, hospital readmissions, and prolonged hospital stays.
Strength of recommendation: Moderate recommendation (B). It is recommended to proactively identify and manage malnutrition in patients with COPD, given its clinical impact on multiple relevant outcomes, even in the presence of a normal or elevated BMI.
Quality of lifeMalnutrition is also associated with quality of life in COPD. This has been assessed using both disease-specific questionnaires—such as the St. George's Respiratory Questionnaire (SGRQ) [18,26,78–80], the Clinical COPD Questionnaire (CCQ) [81], and the COPD Assessment Test (CAT) [4,33,82]—as well as generic tools like the SF-36 Health Survey [78] and the EQ-5D-5L [47]. A BMI<20kg/m2 or <21kg/m2 has been correlated in many of these studies with worse quality of life outcomes, both in overall scores and in specific domains (symptoms, mental state, and functional status) [26,78,80,82]. A meta-analysis of 49 studies found that malnutrition, defined in most cases by low body weight, is associated with poorer quality of life [62]. Likewise, obesity has also been linked to worse quality of life in people with COPD [83]. Weight loss, reduced calf circumference [41,47], low fat-free mass index (FFMI), and its decline over time have been associated with worse scores in the CAT and other quality-of-life assessments [18,33,42,67,82]. Few studies have reported no differences in quality of life between underweight and normal-weight patients [79], but it has been noted that the negative association with low weight disappears in individuals with preserved FFMI. The link between malnutrition and poorer quality of life appears to be independent of the degree of pulmonary impairment [81], suggesting that nutritional therapy may improve quality of life in COPD patients regardless of the level of airway obstruction [4,82].
Level of evidence: 2+. The evidence is based on observational studies (cohorts, meta-analyses), which show a significant association between malnutrition (primarily defined by a low BMI) and poorer quality of life in patients with COPD.
Strength of recommendation: Moderate recommendation (B). Nutritional intervention is recommended in patients with COPD to improve their quality of life, regardless of the degree of pulmonary obstruction. The assessment of parameters such as BMI, FFMI, and calf circumference could be incorporated into routine clinical practice to identify patients at risk.
Exercise toleranceMalnutrition is associated with metabolic and structural changes in both peripheral and respiratory muscles, worsening preexisting dyspnea and exercise intolerance [78]. A BMI below 20kg/m2 has been linked to increased dyspnea [20,29,80,81,84], as measured by the oxygen cost diagram [79]. FFMI and arm circumference are also significantly correlated with dyspnea, respiratory muscle function, and FEV1 [20,29,85].
Exercise capacity, most commonly assessed through the 6-minute walk test (6MWT) [20,21,33,86], is reduced in patients with low body weight and muscle depletion, and shows a significant correlation with FFMI [18,20,21,33,80,86–88]. An association has also been found between low FFMI and poorer performance on the 12-minute walk test [42]. In a case-control study evaluating quadriceps dysfunction, malnutrition—along with inactivity and airflow limitation—was identified as a contributing factor [87].
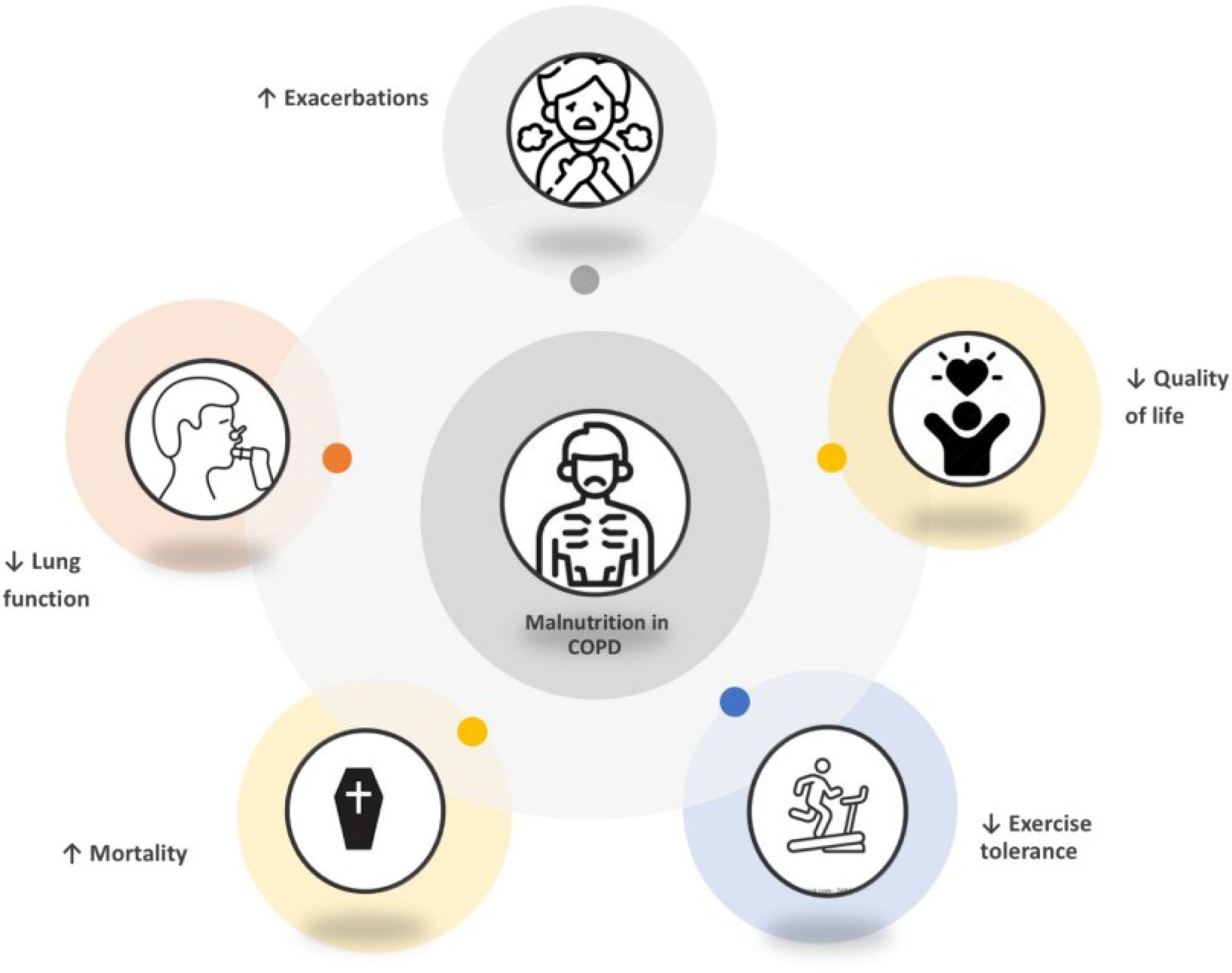
Level of evidence according to SIGN: 2+. Most studies on malnutrition in patients with COPD are observational, particularly cohort studies, along with some case-control analyses. These studies assess the relationship between malnutrition—defined by a low BMI (<20kg/m2) and muscle mass loss measured by FFMI—and increased risk of exacerbations, prolonged hospital stays, readmissions, reduced physical capacity, and poorer quality of life (Fig. 2). Evidence also shows that patients with certain micronutrient deficiencies experience worse quality of life and greater respiratory difficulty, which negatively impacts their exercise capacity. However, some studies suggest that obesity may have a protective effect in certain contexts, although this is not consistent across all evaluated populations.
Strength of recommendation: Moderate recommendation (B). Routine assessment of nutritional status, including BMI and FFMI, is recommended in patients with COPD, as malnutrition has been associated with a higher risk of unfavorable disease progression. There is a well-established association between low BMI, weight loss, and muscle mass loss with increased frequency of exacerbations and hospital readmissions in COPD patients, although the role of obesity as a protective factor is less consistent.
What nutritional assessments should be conducted in a patient with COPD?Weight-related parameters: BMI and weight lossAs mentioned in the previous question, BMI is associated with numerous clinical variables in COPD [48,61,62,71,76,83]. A low BMI is also a risk factor for osteoporosis [89–93], with a cutoff point of BMI<20.5kg/m2 established to identify those patients at higher risk (Thai population) [89]. In this regard, it has been reported that an increase of 1kg/m2 in BMI reduces the risk of osteoporosis [92] (Supplementary 1 Table 5).
Similarly, weight evolution over time is also important, as previously mentioned. Moreover, individuals who have experienced weight loss during the previous year show a 15–20% increase in resting energy expenditure measured by indirect calorimetry compared to the theoretical value estimated by equations. This is partly due to increased consumption associated with respiratory muscles, which are less efficient. However, total energy expenditure may be similar, due to lower energy expenditure from physical activity [94–97].
Level of evidence: 1+. The presence of a meta-analysis linking BMI with the risk of osteoporosis in patients with COPD increases the quality of the evidence. This is further supported by the consistency of observational studies and reviews that endorse the use of anthropometric parameters (such as BMI and FFMI), nutritional screening scales, and body composition analysis tools.
Strength of recommendation: Strong recommendation (A). It is recommended to regularly assess the BMI of patients with COPD, as maintaining an adequate BMI helps improve clinical outcomes and reduce risks. This evaluation allows for the timely identification of those at risk of malnutrition, which is associated with multiple negative clinical outcomes, including osteoporosis, frequent exacerbations, and poorer quality of life.
Nutritional screeningThe most widely evaluated nutritional screening tool in people with COPD is the Mini Nutritional Assessment–Short Form (MNA-SF) (Supplementary 2 Table 1), which is specific to populations over 65 years of age. It has been shown to predict exacerbations [77] and to better identify individuals with osteoporosis than BMI alone, with a 59% prevalence of osteoporosis among those with a positive screening result [93]. Other screening methods, such as the Malnutrition Universal Screening Tool (MUST) (Supplementary 2 Table 2), the Nutritional Risk Screening 2002 (NRS-2002) (Supplementary 2 Table 3), or the adapted screening by Elmore et al. [98], have not yet demonstrated sufficient detection capacity [9,99].
Level of evidence: 2+. The recommendation is based on cohort studies and case-control analyses evaluating the usefulness of different nutritional screening tools in COPD patients, particularly in those over 65 years of age.
Strength of recommendation: Weak recommendation (C). Nutritional screening is recommended for patients with COPD. Among the available tools, the MNA-SF helps identify individuals over 65 years old at higher risk of exacerbations and osteoporosis. Although the MNA-SF shows some superiority in the geriatric COPD population, particularly in detecting the risk of exacerbations and osteoporosis, comparative evidence with other methods remains limited and is mainly based on observational studies.
Diagnosis of malnutritionThere are various criteria for establishing the diagnosis of disease-related malnutrition; however, international scientific societies currently advocate for standardizing this diagnosis through the GLIM criteria. Patients diagnosed with malnutrition according to these criteria have been shown to have higher mortality, readmission rates, and hospital stays, as previously discussed.
Other diagnostic criteria previously used have also been evaluated against the Subjective Global Assessment (SGA) as the reference standard. When comparing the criteria of the Academy of Nutrition and Dietetics and the American Society for Parenteral and Enteral Nutrition (AND-ASPEN), the European Society for Clinical Nutrition and Metabolism (ESPEN), and the GLIM in individuals hospitalized for COPD exacerbation, the AND-ASPEN definition shows the best agreement and accuracy, followed by the GLIM criteria. The AND-ASPEN and ESPEN definitions are also capable of identifying individuals at risk of prolonged hospital stays (9. The Patient-Generated SGA (PG-SGA) has also shown good accuracy, and patients with a score≥9 tend to have lower oxygen saturation, BMI, and functional capacity [100]. The diagnostic capacity of malnutrition using BMI compared to the SGA remains controversial [48,101].
Level of evidence: 1+. This recommendation is based on high-quality comparative studies that have evaluated the diagnostic performance of different criteria and tools in the COPD population.
Strength of recommendation: Moderate recommendation (B). The use of GLIM criteria is recommended for the diagnosis of malnutrition in patients with COPD. There is consistent evidence supporting the superiority of certain diagnostic criteria (AND-ASPEN, GLIM) over others traditionally used, although further standardization in clinical implementation is still needed.
Body compositionAs previously mentioned, the assessment of body composition has shown a relationship with clinical outcomes in patients with COPD, suggesting a worse prognosis when there is muscle mass depletion. Supplementary 1 Table 6[159] shows several studies that evaluate body composition using different techniques. Anthropometry and BIA show good reproducibility in individuals with stable COPD [102] and good correlation with dual-energy X-ray absorptiometry (DXA), which is the gold standard technique [103–106]. The most commonly evaluated body composition parameter is the fat-free mass index (FFMI). It should be noted that formulas used to estimate FFMI through anthropometric measurements tend to overestimate it compared to DXA (by 0.62–1.70kg) [102,103] and BIA (by 0.31–4.4kg); however, some formulas may also underestimate it [107,108].
In terms of anthropometry, individuals with stable COPD and sarcopenia can be identified using mid-upper arm circumference (MUAC) (men<26cm, women<27.5cm) or mid-arm muscle area (MAMA) (men<32.5cm2, women<31.7cm2) [109]. Likewise, reduced calf circumference is associated with adverse clinical outcomes [41]. This is a simple assessment tool that can be used in clinical practice when advanced body composition techniques are not available.
There is a method for estimating lower limb lean volume (LLV) using anthropometry based on limb circumference and skinfolds [110], which has shown good agreement with DXA in patients with moderate-to-severe airflow obstruction and correlates with functionality. A height-adjusted LLV cutoff of ≤9.2L/m has been established to identify malnourished individuals [111]. Attempts to estimate the cross-sectional area of thigh muscle groups using circumferences and skinfolds have not demonstrated strong diagnostic capability [112].
With BIA, the regression formula used to calculate body composition from electrical data is crucial, as it assumes a constant hydration state [105,106]. No ideal formula exists, not even those specifically developed for COPD populations [103,105,107]. Some studies suggest that the formula by Kyle et al. shows better correlation with DXA [104], while others indicate that those by Kulkarni and Deurenberg provide better estimates [108]. To avoid issues with regression equations, it is currently recommended to use raw electrical measurements, as these are associated with higher mortality, worse functionality, and respiratory impairment—more so than FFMI, as previously mentioned.
Patients with COPD experience greater peripheral muscle impairment, particularly in the lower limbs [45,113,114], especially in severe cases, with no significant differences in upper limb or trunk muscle mass [113]. Regional muscle mass loss correlates with airflow obstruction and is associated with increased mortality [45,115].
The cross-sectional area and thickness of the rectus femoris (RF), assessed by ultrasound, correlate with muscle mass variables measured by other techniques (CT and BIA) and with functional capacity. Consistent with greater involvement of the lower limb muscles, RF cross-sectional area is reduced compared to healthy controls with similar FFMI [116–118].
Low FFM determined by anthropometry is a risk factor for osteoporosis, and an increase of 1kg/m2 in FFMI reduces this risk [92].
COPD patients also show altered fat distribution. CT imaging reveals that those with FEV1<80% have increased visceral fat accumulation and intramuscular fat infiltration compared to those with mild obstruction or no COPD, which correlates with markers of metabolic syndrome and coronary artery calcification [119].
Level of evidence: 2++. Based on well-designed cohort studies linking body composition parameters with clinical outcomes in COPD, and on comparative studies between evaluation techniques.
Strength of recommendation: Moderate recommendation (B). It is recommended to assess body composition in patients with stable COPD to identify muscle depletion and guide nutritional support interventions. There is solid evidence supporting the evaluation of body composition as part of the clinical management of COPD patients, particularly due to its prognostic value. However, variability among techniques and formulas, as well as limited equipment availability, may restrict its systematic use.
Functional measures related to nutritionThe most studied functional measures for strength are handgrip dynamometry (HGS) and quadriceps maximal voluntary contraction (QMVC), both of which have shown good reliability and reproducibility [120–124]. Reduced QMVC and HGS are risk factors for mortality in individuals with COPD [125–127] and are associated with muscle mass loss [125,128,129] and poorer quality of life [125]. Their association with the strength of other muscle groups is more debated [45,130]. Quadriceps strength also correlates with the distance covered in the 6-Minute Walk Test (6MWT) [131], with cutoffs to detect exercise intolerance of QMVC<26.2kg and QMVC/m2<9.6kg/m2[132]. The relationship between QMVC and FEV1 remains controversial [45,87,129]. The Five-Repetition Sit-to-Stand Test (5STS) has also been validated for assessing strength and can be used for sarcopenia screening [133].
The most widely used functional test in COPD patients is the 6MWT [134]. Other tests, such as the 10-Repetition Sit-to-Stand Test (10STS), the 30-Second Sit-to-Stand Test (30STS), and the Short Physical Performance Battery (SPPB), show good reproducibility [123] and are associated with low muscle mass estimated by DXA. The most discriminatory cutoffs are 15 repetitions in the 30STS and 10 points in the SPPB [135]. Additionally, the Timed Up and Go test, the Berg Balance Scale, and the unipedal stance test correlate well with QMVC and the 6MWT [136]. The 2-Minute Walk Test is a reliable and validated technique compared with the 6MWT to assess exercise capacity and may be of interest due to its simplicity and better tolerability [137].
Various functional assessments can be performed in hospitalized COPD patients, but few studies have analyzed their validity and reliability [138].
Level of evidence: 2++. Based on well-designed cohort studies linking body composition parameters with clinical outcomes in COPD, and on comparative studies of evaluation techniques.
Strength of recommendations: Moderate recommendation (B). The use of handgrip dynamometry, QMVC, and the 6MWT is recommended as key functional measures to monitor muscle strength and exercise tolerance in COPD patients. There is strong evidence supporting the inclusion of body composition assessment as part of the clinical approach to COPD, especially due to its prognostic value. However, variability in techniques and formulas, as well as equipment availability, may limit systematic application.
Biochemical markers related to nutritionMarkers related to nutritional homeostasisSupplementary 1 Table 7[160–166] presents the main markers related to nutritional homeostasis. Although some have been proposed, none has achieved a sufficient level of evidence to recommend routine clinical use.
Inflammatory variablesHigh-sensitivity C-reactive protein (hs-CRP) and elevated blood levels of interleukin-6 (IL-6) are negatively associated with BMI and functional variables such as the 6MWT [139,140]. Increased levels of CRP and pro-inflammatory cytokines such as tumor necrosis factor-α (TNF-α) and IL-6 are also risk factors for osteoporosis and deterioration of trabecular bone microarchitecture (Trabecular Bone Score) in these patients. A CRP cutoff>2.3mg/L has been established to identify individuals at higher risk of osteoporosis [89–92] (Supplementary 1 Table 4). Albumin levels decrease in inflammatory states, and COPD patients tend to have reduced levels of this biomarker [141], which is also a risk factor for hypercapnic respiratory failure [142].
Level of evidence: 2+. Based on observational studies that have identified consistent associations between inflammatory biomarkers (CRP, IL-6, TNF-α, albumin) and adverse clinical outcomes in COPD patients.
Strength of recommendations: Weak recommendation (C). It is suggested to include the measurement of high-sensitivity CRP and/or serum albumin levels as part of the inflammation phenotype criterion in the GLIM criteria, as well as markers of disease severity to inform prognosis. Although the evidence is mainly observational, the use of these markers may provide relevant clinical information in certain contexts, particularly for evaluating phenotypic inflammation and metabolic-bone risk.
Question 3. Are nutritional interventions effective in patients with COPD?RationaleThe impact of nutritional status on the overall condition of patients with COPD is mainly reflected in weight loss and muscle mass depletion [143]. In patients with COPD and malnutrition, decreased pulmonary function and accelerated disease progression can be expected [144,145]. In addition, patients with COPD often exhibit peripheral muscle dysfunction and atrophy, manifested as reduced muscle strength and endurance [146]. In clinical practice, tailored basic nutrition and oral nutritional supplements are the first-line therapeutic options for patients at risk of malnutrition [147]. Growing evidence supports the positive impact of nutritional supplements—especially those providing additional calories and/or protein or enriched diets—on the nutritional and clinical outcomes of COPD patients.
Based on the available evidence, the panel analyzed the effects of nutritional interventions according to the outcomes described below (Supplementary 1 Table 8).
Pulmonary functionA systematic review that included 13 studies with 916 COPD participants evaluated various nutritional strategies, including protein supplementation, increased fruit and vegetable intake, and higher macronutrient consumption. The authors reported that a prolonged increase in fruit and vegetable intake may positively impact pulmonary function in COPD patients [148]. However, in another systematic review including 11 studies (1183 patients) assessing vitamin D supplementation, no improvement in pulmonary function was observed [149].
AnthropometryA systematic review and meta-analysis of 32 randomized controlled trials (1414 participants) examined the effects of oral energy/protein supplements or enriched diets for at least one week [143]. Nutritional interventions were associated with increases in body weight (mean difference: 1.44kg, 95% CI: 0.81–2.08) and fat-free mass (SMD=0.37, 95% CI: 0.15–0.59). The certainty of the evidence ranged from very low to low.
In COPD patients with sarcopenia, a recent systematic review of 29 RCTs involving 1625 participants (mean age: 67.9±7.8 years) showed that nutritional interventions significantly improved body weight (mean difference: 1.33kg, 95% CI: 0.60–2.05) and FFMI (mean difference: 0.74kg/m2, 95% CI: 0.21–1.27) [150].
Muscle strengthIn the same meta-analysis of 32 RCTs (1414 participants), nutritional interventions including protein supplementation or enriched diets improved handgrip strength (SMD=0.39, 95% CI: 0.07–0.71) [143]. However, in the review of 1625 COPD patients with sarcopenia, nutritional interventions did not significantly improve handgrip strength or quadriceps muscle strength compared to controls [150].
Physical capacityAmong COPD patients with sarcopenia, the same systematic review of 29 RCTs (1625 participants, mean age 67.9±7.8 years) found an improvement in the 6-Minute Walk Test (6MWT) by 19.4 meters (95% CI: 4.91–33.94) in those receiving nutritional supplementation compared to the control group [150]. In a separate systematic review with meta-analysis including 11 studies (287 patients), nitrate supplementation improved exercise capacity (SMD=0.38, 95% CI: 0.04–0.72) [151].
Other outcomesA systematic review including 11 studies (1183 patients) focused on vitamin D supplementation and found no significant differences in exacerbation rate, hospital stay duration, or mortality [149].
RecommendationThe panel recommends nutritional interventions in COPD patients with malnutrition (strong recommendation, moderate level of evidence).
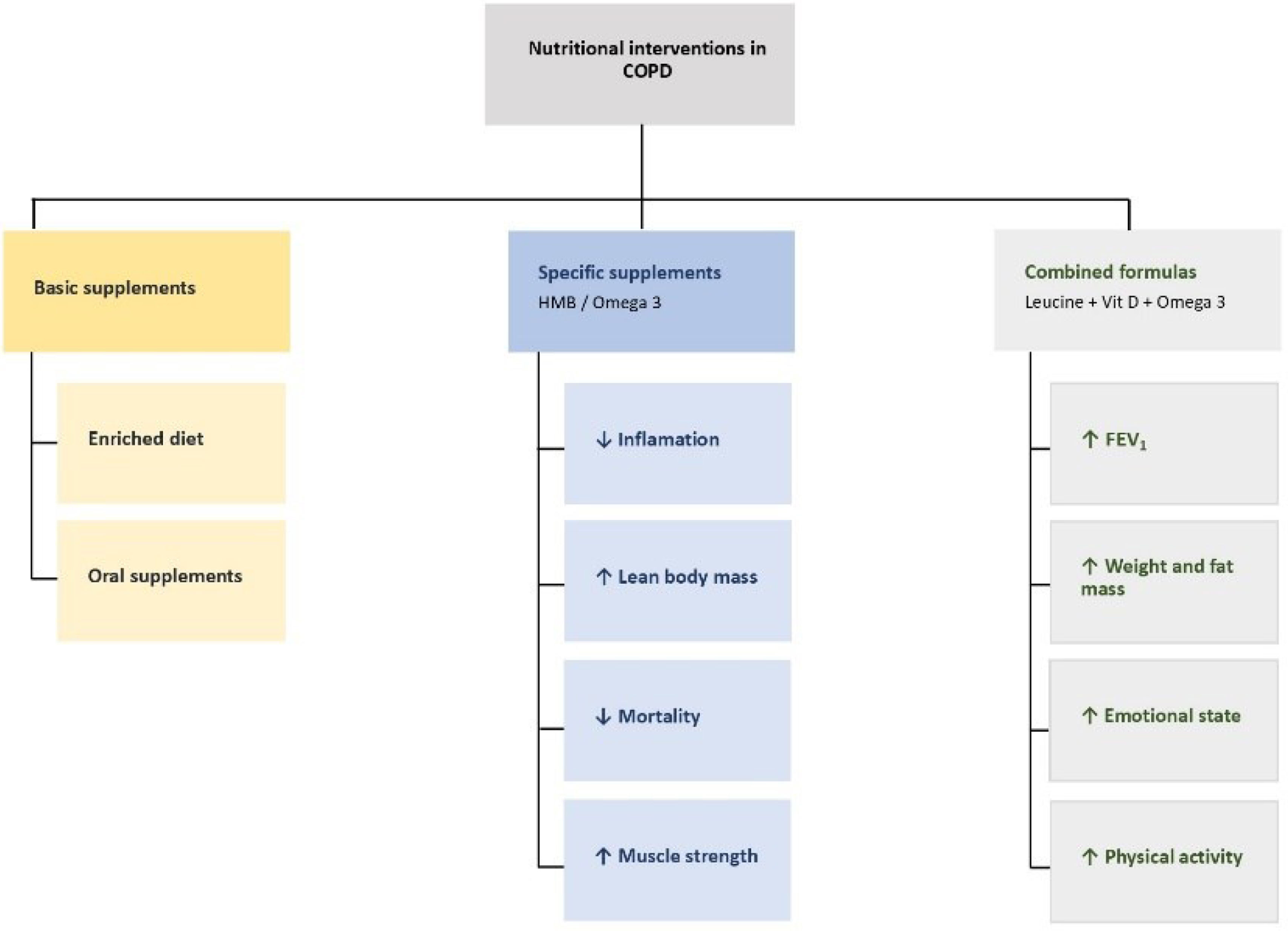
Specific supplementationProposed nutritional interventions for COPD patients are detailed below (see Fig. 3).
Supplementation with β-hydroxy-β-methylbutyrate (HMB)Mortality and nutritional and functional parametersHMB supplementation in hospitalized COPD patients has shown significant clinical benefits, including reduced mortality and improved nutritional and functional parameters, based on a post hoc analysis of the NOURISH study [153]. Administering 3g/day of HMB combined with protein during hospitalization and for 90 days post-discharge reduced mortality risk by 71% compared to placebo. This effect was statistically significant at 30, 60, and 90 days post-discharge.
Furthermore, it has been described that patients supplemented with HMB present a significant increase in hand grip strength, a key marker of muscle function and nutritional status.
Patients supplemented with HMB also showed significant improvements in handgrip strength, a key marker of muscle function and nutritional status. Furthermore, significant weight gain was observed at discharge (+0.61kg vs. −0.09kg with placebo), along with improvements in nutritional biomarkers such as hemoglobin, serum calcium, and vitamin D.
Recommendation
Early administration of HMB supplementation during hospitalization and continued post-discharge reduces mortality and improves nutritional parameters in COPD patients.
InflammationTargeted nutritional supplementation with HMB and omega-3 fatty acids (EPA/DHA) has shown significant benefits in managing COPD patients with FEV1<80%, especially those with muscle mass loss, systemic inflammation, or metabolic alterations [152]. The combination of HMB (3g/day) with EPA/DHA produced greater effects than omega-3 alone, including reductions in visceral fat and inflammatory markers such as IL-6, IL-12, and TNF-β.
Recommendation
Supplementation with HMB and EPA/DHA in COPD patients is a safe and effective nutritional strategy to improve body composition, reduce systemic inflammation, and optimize clinical outcomes.
Combined use of high-calorie/high-protein supplements with 3.6g leucine, vitamin D, omega-3, and polyunsaturated fatty acids [154,155]These formulas have shown improvements in body weight and fat mass in COPD patients. Some studies reported FEV1 improvement, especially when combined with physical exercise. One randomized, placebo-controlled prospective study also found improvements in mood, inspiratory muscle strength, and daily step count. However, in cachectic patients with moderate COPD, long-term physical capacity did not improve.
Recommendation
Enriched formulas containing leucine, vitamin D, omega-3, and polyunsaturated fatty acids may offer additional benefits beyond weight gain, such as improvements in FEV1, inspiratory muscle strength, and mood. More studies are needed to confirm these findings.
Omega 3 (EPA y DHA)Administering 2g/day of EPA/DHA for 10 weeks improves protein metabolism, increases lean body mass, and reduces specific inflammatory markers in COPD patients. Its use is recommended in stable COPD patients with sarcopenia, muscle loss, or persistent systemic inflammation.
Recommendation
Consider using EPA/DHA supplementation in stable COPD patients with sarcopenia, muscle loss, or chronic systemic inflammation. As mentioned in Section 3.2.1, combining it with HMB may further enhance clinical outcomes.
Other formulationsVarious formulations have been studied, including normocaloric/normoprotein formulas enriched with omega-3 and -6 and prebiotic fiber; high-protein formulas with omega-3 and vitamins A, C, and E; or hypocaloric, high-protein formulas enriched with magnesium and vitamin C. However, due to the small number of patients included in these studies, no strong conclusions can be drawn [156–158].
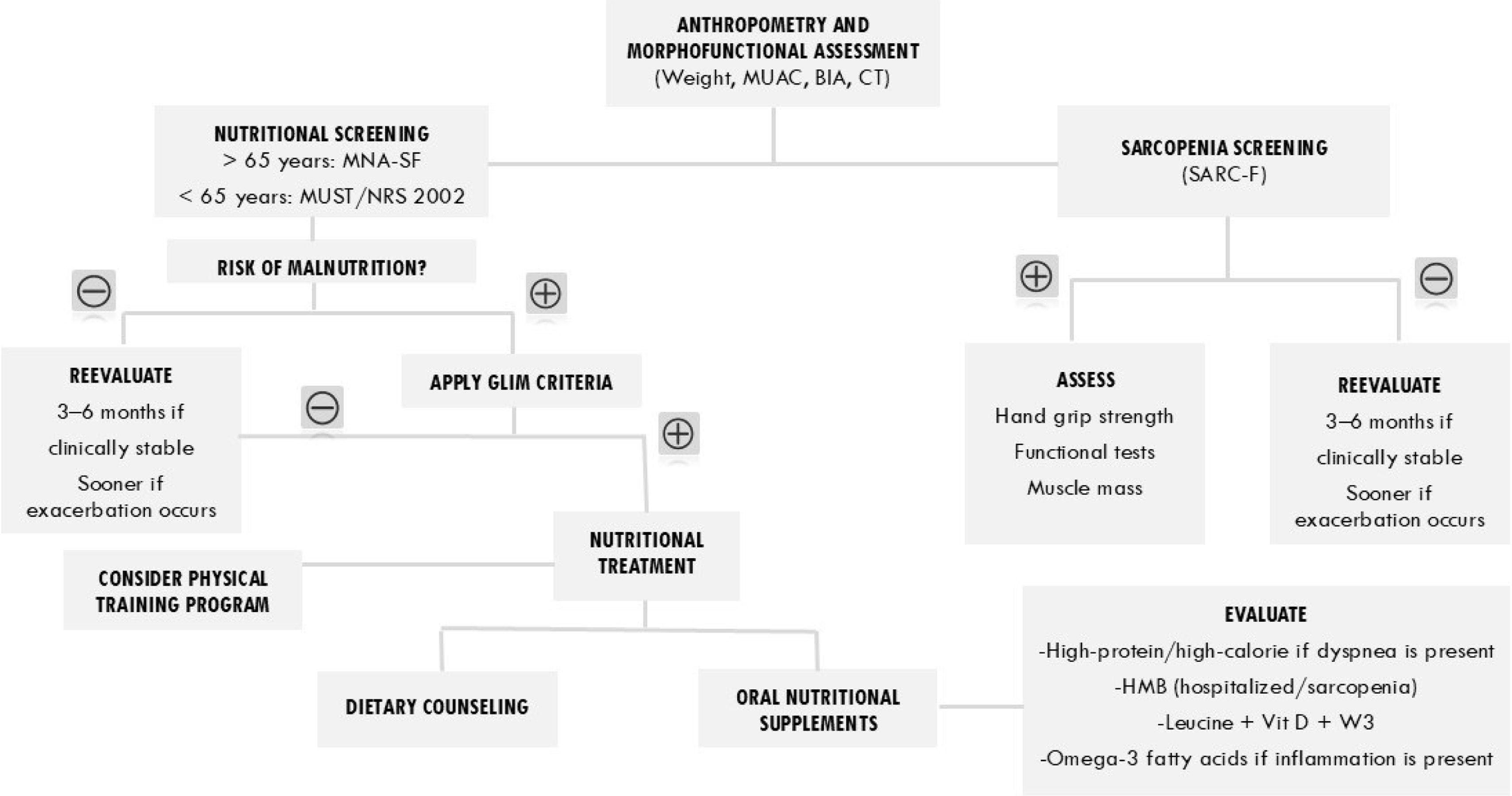
Based on the above, a nutritional assessment algorithm for COPD patients is proposed (Fig. 4). (Table 1).
Nutritional assessment algorithm in patients with COPD. Abbreviations: AC: arm circumference; BIA: bioelectrical impedance analysis; CT: computed tomography; GLIM: Global Leadership Initiative on Malnutrition; HMB: beta-hydroxy-beta-methylbutyrate; MNA-SF: Mini Nutritional Assessment – Short Form; MUST: Malnutrition Universal Screening Tool; NRS 2002: Nutritional Risk Screening 2002; SARC-F: tool proposed to identify individuals at risk of sarcopenia; Vit D: vitamin D; W3: omega-3.
Summary of recommendations for the three questions included in the position statement.
| 1. What is the impact of malnutrition on the clinical and functional progression of patients with COPD? | |
|---|---|
| Dimension | Recommendation (Level of evidence, strength of recommendation) |
| Pulmonary function | It is recommended that nutritional status be regularly monitored in patients with COPD, as evidence suggests that malnutrition may be associated with worsening pulmonary function (2+, B) |
| Mortality | It is recommended to initiate nutritional strategies aimed at improving weight status as early as possible in COPD patients with low BMI (<20kg/m2) (2+, B) |
| Exacerbations | It is recommended to proactively identify and manage malnutrition in patients with COPD, given its clinical impact on multiple relevant outcomes, even in the presence of a normal or elevated BMI (2+, B) |
| Quality of life | Nutritional intervention is recommended in patients with COPD to improve their quality of life, regardless of the degree of pulmonary obstruction. The assessment of parameters such as BMI, FFMI, and calf circumference may be incorporated into routine clinical practice to identify patients at risk (2+, B) |
| Exercise tolerance | Routine assessment of nutritional status, including BMI and FFMI, is recommended in patients with COPD, as malnutrition has been associated with a higher risk of unfavorable disease progression (2+, B) |
| 2. What nutritional assessments should be conducted in a patient with COPD? | |
|---|---|
| Dimension | Recommendation (Level of evidence, strength of recommendation) |
| Weight-related parameters | It is recommended to regularly assess the BMI of patients with COPD, as maintaining an adequate BMI helps improve clinical outcomes and reduce risks (1+, A) |
| Nutritional screening | Nutritional screening is recommended for patients with COPD. Among the available tools, the MNA-SF helps identify individuals over 65 years of age who are at higher risk of exacerbations and osteoporosis. |
| Diagnosis of malnutrition | The use of GLIM criteria is recommended for the diagnosis of malnutrition in patients with COPD (1+, B) |
| Body composition | It is recommended to assess body composition in patients with stable COPD to identify muscle depletion and guide nutritional support interventions (2++, B) |
| Functional measures | It is recommended to assess handgrip dynamometry, QMVC, and the 6MWT as key functional measures to monitor muscle strength and exercise tolerance in COPD patients (2++, B) |
| Biochemical markers | It is suggested to include the measurement of high-sensitivity CRP and/or serum albumin levels as part of the inflammation phenotype criterion in the GLIM criteria, as well as markers of disease severity to inform prognosis (2+, C) |
| 3. Are nutritional interventions effective in patients with COPD? | |
|---|---|
| Dimension | Recommendation |
| Specific supplementation | The panel recommends nutritional interventions in COPD patients with malnutrition (moderate level of evidence, strong recommendation).Specific recommendations:1. Early administration of HMB supplementation during hospitalization and its continued post-discharge reduces mortality and improves nutritional parameters in COPD patients.2. Supplementation with HMB and EPA/DHA in COPD patients is a safe and effective nutritional strategy to improve body composition, reduce systemic inflammation, and optimize clinical outcomes.3. Enriched formulas containing leucine, vitamin D, omega-3, and polyunsaturated fatty acids may offer additional benefits beyond weight gain, such as improvements in FEV1, inspiratory muscle strength, and mood.4. EPA/DHA supplementation should be considered in stable COPD patients with sarcopenia, muscle loss, or chronic systemic inflammation. |
Abbreviations: 6MWT: six-minute walk test; BMI: body mass index; COPD: Chronic Obstructive Pulmonary Disease; CRP: C-reactive protein; DHA: docosahexaenoic acid; EPA: eicosapentaenoic acid; FEV1: forced expiratory volume in the first second; FFMI: fat-free mass index; GLIM: Global Leadership Initiative on Malnutrition; HMB: β-hydroxy β-methylbutyrate; MNA-SF: Mini Nutritional Assessment – Short Form; QMVC: Quadriceps Maximum Voluntary Contraction.
Malnutrition and sarcopenia are common conditions in COPD patients and represent poor prognostic factors, as they worsen functional capacity, increase morbidity, and even mortality.
The lack of well-designed studies generating strong evidence justifies the development of algorithms for diagnosing and managing malnutrition and sarcopenia in these patients. In this consensus, developed by SEPAR and SEEN, the importance of screening for both conditions is emphasized, as well as the need for a proactive approach through dietary recommendations, lifestyle changes, and the use of oral nutritional supplements, aiming to improve clinical outcomes in patients with COPD.
Author contributionsJMD, JPSL, JMFG, and IBL were the coordinators of the document. All authors contributed equally to its preparation.
Use of artificial intelligenceNo artificial intelligence tools were used in the preparation of this manuscript.
FundingThis manuscript did not receive any funding.
Conflicts of interestJMD has received fees for lectures and funding for conference attendance from AstraZeneca, BIAL, Boehringer Ingelheim, Chiesi, FAES, Fresenius-Kabi, Gebro, GSK, Janssen, Menarini, Novartis, Sanofi, Roche, Teva, Pfizer, and Zambon.
JPSL has received fees for lectures and funding for conference attendance from Abbott Nutrition Laboratories, Nutricia, Fresenius-Kabi, Nestlé HealthScience, and Adventia Pharma.
JMFG has received fees for lectures and funding for conference attendance from Esteve Laboratories, MundiPharma, AstraZeneca, Boehringer Ingelheim, Ferrer, Menarini, Rovi, GSK, Chiesi, Novartis, and Gebro Pharma.
IBL has received fees for lectures and funding for conference attendance from Abbott Nutrition Laboratories, Nutricia, Fresenius-Kabi, Nestlé HealthScience, Adventia Pharma, Vegenat, Persan Farma, Novo Nordisk, and Lilly.
RTC has no conflicts of interest related to the publication of this document.
RG has received fees for lectures and funding for conference attendance from AstraZeneca, GSK, Novartis, FAES, Chiesi, Mundipharma, Menarini, TEVA, Grifols, Ferrer, Boehringer Ingelheim, Rovi, and Gebro.
NCIH has received fees for lectures from Abbott Nutrition Laboratories, Cantabria Labs, Vegenat, Nutricia, Fresenius-Kabi, Nestlé HealthScience, and funding for conference attendance from Abbott Nutrition Laboratories, Vegenat, Nutricia, Fresenius-Kabi, Nestlé HealthScience, and Persan Farma.
CAAD has no conflicts of interest related to the publication of this document.
YGD has received fees for lectures from Abbott Nutrition Laboratories, Nutricia, Fresenius-Kabi, Nestlé HealthScience, and funding for conference attendance from Abbott Nutrition Laboratories, Nutricia, Fresenius-Kabi, Nestlé HealthScience, and Persan Farma.
AAO has received fees for lectures and funding for conference attendance from AstraZeneca Laboratories, Chiesi, FAES, Grifols, GSK, and Menarini.
MDAG has no conflicts of interest related to the publication of this document.
PCR has received fees for lectures and scientific advisory services over the past three years from Janssen, Menarini, and Vivisol; and funding for attendance at congresses from Boehringer Ingelheim, Menarini, and Vivisol.
MRF has received fees for lectures and funding for conference attendance from Abbott Nutrition Laboratories, Nutricia, Fresenius-Kabi, Nestlé HealthScience, and Persan Farma.