Cystic fibrosis (CF) is an autosomal recessive, multisystem genetic disease that mainly affects the exocrine glands due to the absence or alteration of a protein, called cystic fibrosis transmembrane conductance regulator (CFTR).1,2 Until a decade ago, the only treatments available tried to control or prevent the symptoms that were occurring. However, in recent years a line of treatments that improve the functionality of the altered protein has been developed, called CFTR modulators. Tezacaftor–ivacaftor (TEZ/IVA) is modulator of CFTR, indicated in a combined administration regimen for the treatment of CF patients≥6 years, homozygous or heterozygous for the F508del mutation with residual function mutations.3 This drug has been available in Spain since October 1, 2019.
The objective of the study was to study the efficacy and safety of the combination TEZ/IVA in real life in adult patients with Cystic Fibrosis belonging to different units in Spain.
We conducted an ambispective, multi-centre, real-life study with 144 adult patients, carried out from 1 December 2019 to 31 December 2021 in the following CF hospitals of Spain: Hospital Universitario Virgen del Rocío in Sevilla (33 patients), Hospital Universitario La Princesa in Madrid (20), Hospital Universitario 12 de Octubre in Madrid (19), Hospital Universitario La Paz in Madrid,11 Hospital Universitario de Cruces in Bilbao (24), Hospital Universitario Central de Asturias,14 Hospital Regional Universitario de Málaga11 and Hospital Universitario de A Coruña.12 For this study, we included patients with a minimum age of 18 years, who met diagnostic criteria for CF4 and received a consecutive dose of 100mg TEZ/150mg IVA in the morning and 150mg IVA in the evening, with a 12-hour interval. Patients who did not complete at least 3 months of treatment were excluded.
We collected the following general clinical variables: age at treatment initiation (age mean of 31.2 yr.±9.5), sex (54.2% male), mutation type (77.1% homozygous F508del or 22.9% heterozygous F508del), exocrine pancreatic insufficiency in 88.3%, chronic bronchial infection in 84.0%, being the most prevalent microorganisms Pseudomonas aeruginosa (40.3%), Staphylococcus aureus (52.8%) and methicillin-resistant Staphylococcus aureus (9.7%).
In addition, we recorded FEV1 and BMI (body mass index) values every 3 months (baseline, 3, 6, 9 and 12 months after treatment) in the clinic visits and a specific questionary of life quality (CFQ-R) every 6 months (baseline, 6 and 12 months). Additionally, we recorded the number of respiratory exacerbations every 6 months. The number of cycles of oral and intravenous antibiotics was also counted.
We compared the variables, lung function, BMI, and quality of life with baseline (drug initiation), while the number of pulmonary exacerbations were compared with 6 and 12 months prior to start the treatment. We collected tolerability and overall drug safety data with the adverse effects observed and whether this had an impact on the decision to discontinue the drug.
The data presented here correspond to the data collection platform of patients on SYMKEVI 12+treatment “VALTERMED” https://valtermed.mscbs.es/ according to the protocol signed by the Ministry of Health and Vertex Pharmaceuticals and are publicly accessible to the entire scientific community and CF units. The document followed STROBE (http://www.strobe-statement.org/) recommendations for observational studies.
A parametric (ANOVA) or non-parametric (Mann–Whitney–Wilcoxon test) tests were performed, depending on whether the assumptions of normality and homoscedasticity were fulfilled. For qualitative variables, comparison of proportions was tested using the χ2 test. All analyses were performed using R5 statistical software.
The comparison of %FEV1 and BMI values at baseline and 3, 6, 9, 12 months after the start of treatment showed an increasing trend, but it was not resulted to be statistical significant (Table 1). In addition, we found that the patients experienced at 12 months post-treatment an increment of 3.4 points in %FEV1 and 0.4kg/m2 in BMI (Table 1).
Variable mean comparisons between baseline and 3, 6, 9 and 12-months post-treatment.
| Development of the parameters | Mean difference obtained in each time collection vs baseline | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Variables | Basal | 3 months | 6 months | 9 months | 12 months | 3 months | 6 months | 9 months | 12 months |
| %FEV1 | 62.6±23.6 | 64.4±22.5 | 66.8±21.8 | 63.4±22.0 | 65.9±22.9 | 1.8 | 4.3 | 0.9 | 3.4 |
| BMI | 21.9±2.9 | 22.3±2.9 | 22.6±5.02 | 22.6±2.9 | 22.2±3.8 | 0.4 | 0.7 | 0.7 | 0.4 |
| CFQ-R respiratory | 60.5±4.9* | ND5 | 72.2±17.3* | ND | 71.2±18.9* | ND | 11.7* | ND | 10.7* |
| CFQ-R digestive | 74.6±19.8 | ND | 80.6±18.6 | ND | 80.8±19.1 | ND | 6.0 | ND | 6.2 |
| CFQ-R vitality | 60.3±23.4 | ND | 69.6±20.1 | ND | 69.5±16.6 | ND | 9.2* | ND | 9.2* |
| CFQ-R physical activity | 65.0±26.8 | ND | 73.8±22.0 | ND | 73.4±17.5 | ND | 8.8 | ND | 8.3 |
| CFQ-R food | 83.3±23.8 | ND | 81.2±24.7 | ND | 79.6±26.9 | ND | −2.0 | ND | −3.6 |
| CFQ-R daily activities | 77.4±23.2 | ND | 80.7±18.9 | ND | 85.4±16.2 | ND | 3.3 | ND | 8.0* |
| CFQ-R treatment | 49.8±19.5 | ND | 55.1±19.8 | ND | 51.9±23.0 | ND | 5.3 | ND | 2.1 |
| CFQ-R emotional | 75.1±20.9 | ND | 76.0±17.1 | ND | 77.3±13.7 | ND | 0.9 | ND | 2.3 |
%FEV1: maximum exhaled volume during the first second of forced exhalation; BMI: body mass index; CFQ-R: Cystic Fibrosis Questionnaire Revised; ND, data not available. The significant difference (p<0.05) of the assessed variables is showed by asterisks.
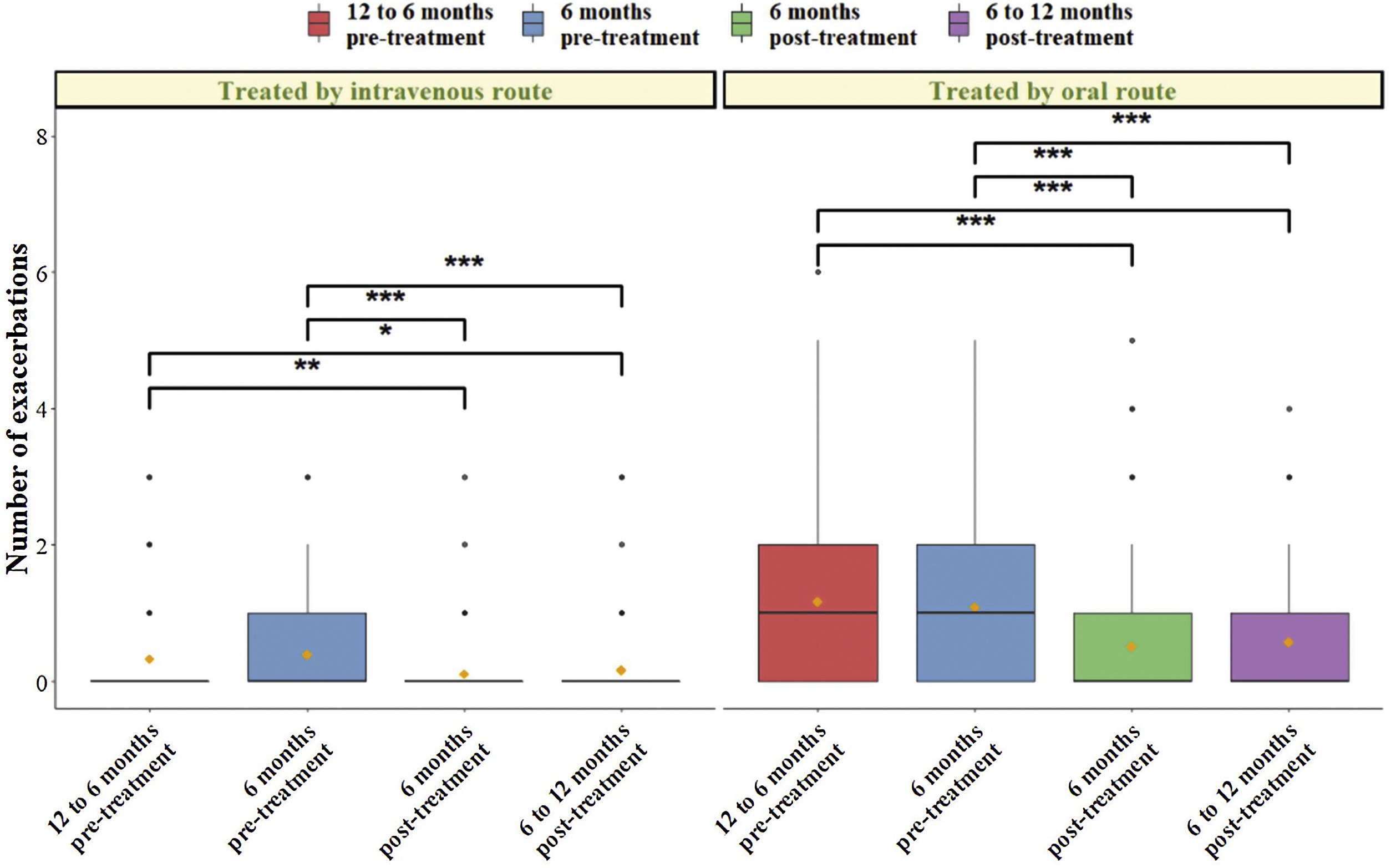
The comparison of baseline scores in the different CFQ-R domains with the scores obtained at 6 and 12 months post-treatment are shown in Table 1. All items improved their baseline score, being statistically significant the change for respiratory domains. In addition, the mean difference obtained for each time collection against baseline showed that in all items except for eating, treatment and emotional items, there was a clinically relevant change of more than four points from the start to the end of treatment. Additionally, the number of respiratory exacerbations at 12 and 6 months against the recorded at 6 and 12 after treated with oral and intravenous antibiotics showed a significant decreased (Fig. 1).
Boxplot with error bars of the number of oral and intravenous exacerbations at 12-6 months and 6 months pre- and post-treatment. The mean of each group is represented by a yellow diamond and median by a black line. The statistical significance level between each group is shown by asterisks (*p<0.05, **p<0.01, ***p<0.001).
Out of the total of 144 patients, only 21 (14.6%) had medication-related adverse effects. The majority were related with the elevation of transaminases (28.6%) and symptoms such as cough (9.5%), abdominal pain (9.5%) and headache (9.5%).
Meanwhile, treatment interruptions occurred in 4 patients (2.8%) due to hepatotoxicity and progressively reintroduced. On the other hand, 11 patients had a definitive interruption due to 2 patients (1.4%) had adverse effects (dyspnea, hepatotoxicity, and depressive mood), 8 (5.5%) lack of efficacy and subsequent switch to the elexacaftor–tezacaftor–ivacaftor combination and 1 (0.7%) voluntary abandonment.
This study is the first in real life to analyze the efficacy and safety of the TEZ/IVA in adult patients with CF, followed in different CF units in Spain, after 12 months of treatment. At present there are few publications that show the results obtained after the use of this modulator drug in daily clinical practice.6
CF is a disease that leads to a progressive deterioration of lung function, with a progressive loss per year of 1.0–3.0% in FEV1.7 Lung function is a very important value in our practice as it helps us make decisions regarding the prescription of treatments. TEZ/IVA there has been a 3.4% increase in %FEV1 after 12 months of treatment.8–10
We have found an increase in BMI at 12 months of treatment, similar data to those found in the 96-week extension study10 and a real-life study with 45 patients of 4 weeks duration.6
In the CFQ-R questionnaire, we observed that 4-point increase in the respiratory, digestive, vitality, physical activity, and daily activities domains.11
Pulmonary exacerbations are events of great importance in CF patients as they worsen quality of life, favoring the progressive decline in lung function and are associated with an increase in mortality.12–14 We have observed in our sample a reduction in the number of exacerbations with the taking of TEZ/IVA, results similar to those obtained in clinical trials.8,10
TEZ/IVA is a generally safe and well-tolerated drug. The most frequent adverse effect in our series is elevation of transaminases.8,15–17
The study has some limitations, it includes only adult patients≥18 years, however, the results we obtained in the parameters evaluated are similar. Likewise, the work includes 144 patients who, although they do not include all Spanish patients of this age group treated TEZ/IVA, constitute approximately 35%, so we consider that it is a representative sample. The development of CFTR modulators marks the beginning of a new era for CF patients.18