Alpha-1 antitrypsin deficiency (AATD) is an autosomal codominant condition, known to predispose to early onset pulmonary emphysema and chronic obstructive pulmonary disease. Generally, lung manifestations of the disease affect smokers at the third or fourth decade of life and so far, intravenous injections of purified alpha-1 antitrypsin are the single target therapy for AATD. Given that lung function in children is typically normal, augmentation therapy has never been systematically studied in pediatric population.
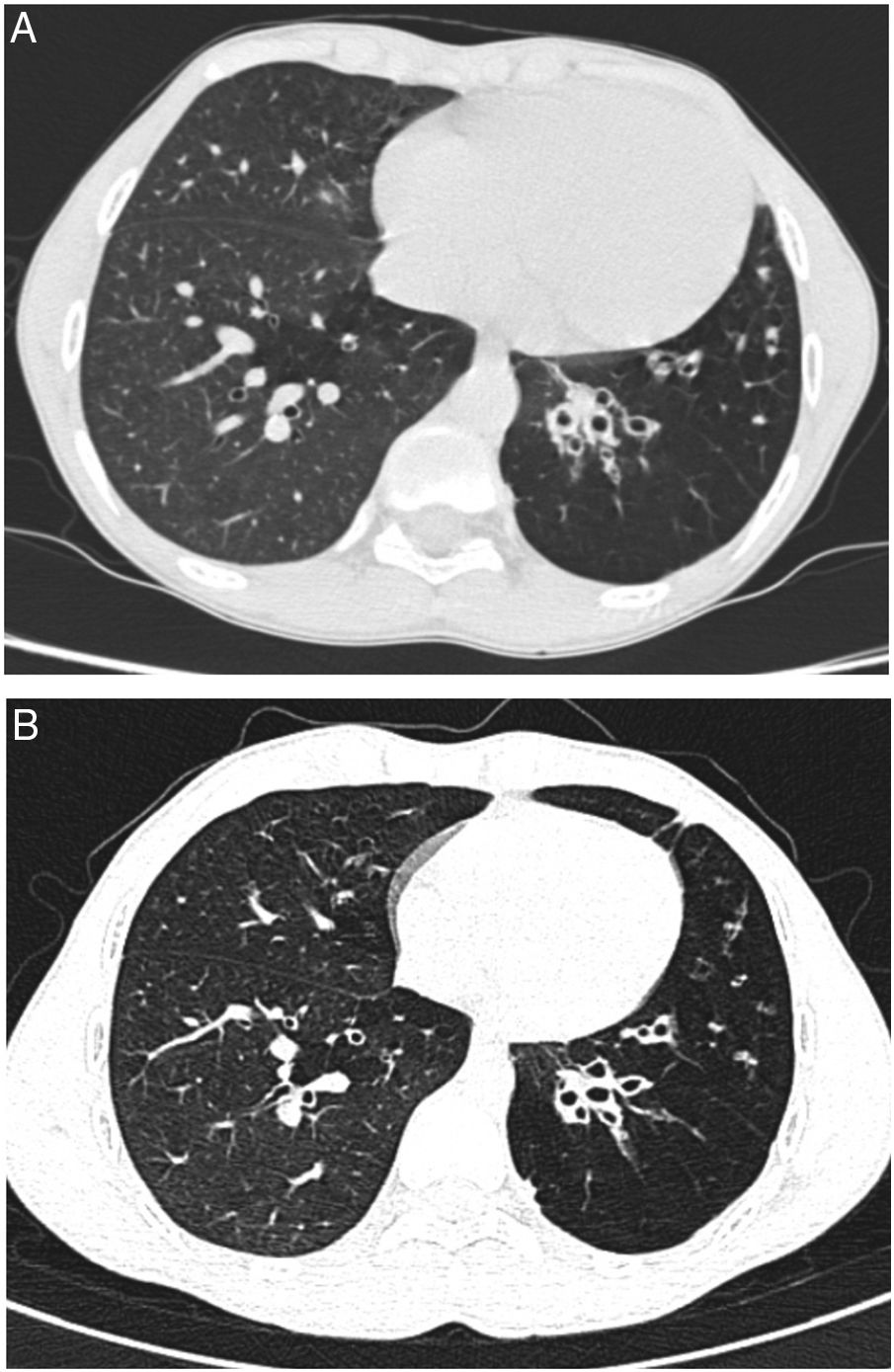
We report a case of a male neonate born at 40 weeks of gestation, weighing 2750g, with an uneventful postnatal period. His first year of life was marked by downward crossing of weight percentile, recurrent suppurative otitis media and two hospitalizations due to respiratory infections. At 9 months of age, routine laboratory investigation revealed anemia (11.8g/dL) and elevated liver enzymes (aspartate transaminase 596U/L, alanine transaminase 722U/L, alkaline phosphatase 239U/L and gamma-glutamyl transpeptidase 75U/L). Due to decreased alpha-1 antitrypsin (AAT) serum concentration (50mg/dL) gene sequencing (SERPINA1) was performed and revealed a ZPlowell genotype. There was no ultrasound evidence of liver impairment. The child's follow-up was interrupted due to a problematic social situation. His family medical history was unremarkable. However, a significant passive smoke exposure due to paternal smoking was reported. He returned to our observation at age 10 years, complaining of recurrent productive coughing, wheezing as well as dyspnea on exertion. On physical examination the patient presented with pale skin, weak appearance and low weight for his age (10th percentile). The chest auscultation revealed diffuse expiratory wheezing and bilateral basal crackles. Pulmonary function tests indicated a non-reversible mild airflow obstruction (FEV1 71.3% pred, FEV1/FVC 74% pred). Laboratory studies showed aggravated anemia, peripheral eosinophilia, elevated total IgE and sensitization to common inhalant allergens (RAST positive). At this time serum level of AAT was 36.7mg/dL. Chest computed tomography (CT) imaging showed lower lobe predominant panlobular emphysema and cystic bronchiectasis (Fig. 1).
He started inhaled therapy with medium-dose corticosteroid (budesonide 160mcg) plus long-acting β-agonist (formoterol 4.5mcg) and leukotriene receptor antagonist (montelukast 10mg). Despite optimized anti-inflammatory and bronchodilator therapy associated with lifestyle preventive measures, the child remained symptomatic with recurrent use of antibiotic therapy owing to respiratory infections. Spirometric monitoring over 1 year demonstrated persistent mild airflow obstruction (FEV1 77.8% pred) and air trapping (RV 181.8% pred). At age 14 years, given the severity of emphysema and the risk of disease progression, and in the absence of therapeutic alternatives, it was decided to start AAT augmentation therapy. Up till now, he has been receiving weekly intravenous injections with 60mg/kg of AAT (Prolastin, Bayer Corporation). So far, 132 infusions have been administered without complications. Regular treatment with inhaled corticosteroids and bronchodilators was left unchanged. Clinically, in the first two years of treatment, the patient has experienced a significant improvement in his overall condition (weight gain up to the 25th percentile) and respiratory symptoms. He had no emergency department admissions nor hospitalizations in the last two years. Functional reassessment at 18 months of treatment showed stable FEV1 and improvement of air trapping (FEV1 77.8% pred; RV 168.9% pred). Stability of pulmonary emphysema and bronchiectasis was observed on high resolution chest CT (Fig. 1).
Studies on the natural history of AATD indicated that emphysema usually begins in the third or fourth decade of life in those exposed to cigarette smoking.1 Although there is paucity of data in the pediatric age group, children and adolescents with AATD have not been shown to have significant lung function abnormalities.2 However, Hird et al. have suggested a tendency to hyperinflation in affected children with liver disease.3 Our patient suffers from severe deficiency due to ZPlowell genotype. Plowell (rs121912714; p.Asp256Val) is a rare SERPINA1 variant that has been associated with exaggerated intracellular degradation of newly synthetized protein and to 24% of normal serum levels. Compound heterozygotes with severe deficient Z allele were reported to have an increased risk of emphysema in adulthood.4 In this case, it is possible that tobacco smoke exposure, coexisting inflammatory disease (atopic asthma), frequent low respiratory tract infections and poor nutritional status have contributed to pulmonary function deterioration and emphysema development at such early age. This case highlights the importance of family-based smoking intervention to attain a nonsmoking lifestyle in children with AATD. It is worth noting that determination of AAT serum concentration should be considered in the panel of tests for investigation of adolescents with respiratory symptoms unresponsive to usual asthma treatment interventions.5,6 In case of AATD, the evaluation must be completed with genotyping and, eventually SERPINA1 sequencing to exclude rare deficiency alleles.
Intravenous administration of AAT is currently the only specific treatment for AATD Evidence from randomized placebo-controlled clinical trials showing that augmentation therapy in adult patients with severe AATD is safe and effective at reducing the progression of emphysema as assessed by CT densitometry,7,8 have supported its recommendation in international guidelines.9 In addition, a large observational study reported decreased mortality and slower FEV1 decline in subjects receiving augmentation therapy as compared with those not receiving therapy.10
We report the second case described in the literature on augmentation therapy for AATD at pediatric age.11 On both cases, a good safety profile and significant clinical benefit were observed. Based on these favorable outcomes, we believe that in selected children with AATD related-lung disease, augmentation therapy may provide the same benefits demonstrated in adults. Therefore, we encourage research groups to include children and adolescents in large AATD clinical trials.