The introduction of immunotherapy in the treatment of non-small cell lung cancer (NSCLC) is changing its pejorative prognosis by the positive impact on the patients’ overall survival. Available immunotherapies in this indication target the PD-1 (programmed cell death 1) receptor overexpressed by the cancer cells and T-lymphocytes (Nivolumab, Pembrolizumab) or its ligand PD-L1 (Durvalumab, Atezolizumab). However, the administration of an anti-PD-1/PD-L1 treatment could be associated with a reactivation of infectious diseases (e.g. tuberculosis, herpes zoster) by the concomitant action on the T-lymphocytes involved in the antimicrobial defence.1 Some rare cases of pulmonary or pleural tuberculosis are reported in patients receiving Pembrolizumab as treatment for different cancers (melanoma, Hodgkin lymphoma, NSCLC, Merkel cell carcinoma).2–5
We describe the case of a pleural effusion occurring three months after the initiation of a treatment by Pembrolizumab in a patient with an advanced NSCLC causing some etiological diagnosis problems and difficulties in the therapeutic management. The final diagnosis was pleural tuberculosis reactivated during the treatment by Pembrolizumab.
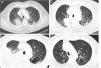
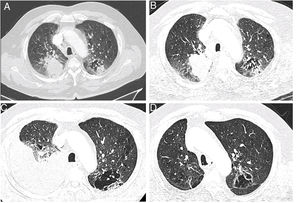
A 75 year old man, retired (previous builder), active smoker (15 packs-year), having a dyslipidaemia and diabetes mellitus treated by oral antidiabetics, presented a haemoptysis in July 2018, allowing the discovery of a right upper lobe lung adenocarcinoma (Fig. 1A) expressing PD-L1 at 2%, with KRAS G12D mutation, classified stage cT3N3M1c (pulmonary and cerebral metastases) according to the 8th edition of the International Association for the Study of Lung Cancer.6 After discussion during a multidisciplinary thoracic oncology meeting (MTOM), a chemotherapy by Carboplatine-Pemetrexed and an encephalic radiotherapy were validated (the chemo-immunotherapy association in 1st line was not allowed at the time). After an initial therapeutic response, the chest computer-tomography scan (CT-scan) realized in January 2019 after four cycles of Carboplatine-Pemetrexed and two maintenance cycles by Pemetrexed found a tumor thoracic progression (Fig. 1B) and the appearance of liver metastases. A treatment by Pembrolizumab was started after discussion at the MTOM. After the 4th cure, the patient presented a progressive dyspnea 2 mMRC7 associated with the abolition of vesicular murmur in the inferior part of the right side of the thorax. An abundant pleural effusion was objectified on the CT-scan realized in April 2019 without other changes on the imaging (Fig. 1C). The thoracentesis evacuated 1700mL of amber exudative liquid (proteins 45g/L, lactate dehydrogenase 279 UI/L) with low glucose level (1.15g/L), predominantly lymphocytic (lymphocytes 90%, plasma cells 7%, macrophages 2%, neutrophils 1%), without atypical cells. The immunohistochemistry showed T-lymphocytes expressing CD3+ and CD5+. Direct microscopy exams found no infectious agents and the absence of acid-fast bacilli. The treatment by Pembrolizumab was continued and the blood lymphocytes count always remained normal (1.5–2g/L). When the culture from pleural liquid became positive for Mycobacterium tuberculosis, 59th days after the thoracentesis, the case was discussed in MTOM to validate the addition of anti-tuberculosis treatment while continuing the immunotherapy given the tumor response. The anti-tuberculosis treatment was realized for 6 months (4 months: Isoniazide 5mg/kg/day, Rifampicine 10mg/kg/day, Pyrazinamide 20mg/kg/day, Ethambutol 15mg/kg/day; 2 mois: Izoniazide, Rifampicine) with a good tolerance and without recurrence of the pleural effusion. The tumor response persists after two years of treatment by immunotherapy (Fig. 1D).
Chest CT-scan images showing the evolution of the patient. (A) Axial contrast section showing an upper right lobe mass (lung adenocarcinoma) and a mixed lesion (cavity and solid) in the upper left lobe (July 27th, 2018). (B) Axial contrast section showing a progression of the upper right lobe mass after chemotherapy; baseline for the start of the immunotherapy (January 18th, 2019). (C) Axial contrast section showing an abundant right pleural effusion (pleural tuberculosis) and previously described mixed lesion in the upper left lobe (April 30th, 2019). (D) Axial contrast section showing an almost complete tumor response after 28 cycles of immunotherapy (March 23rd, 2021).
The pleural effusions appearing in the first weeks after the introduction of a cancer immunotherapy are frequently found on the chest CT-scan realized in emergency (24% of cases).8 Since there are many etiologies, establishing the diagnosis and adopting the best therapeutic strategy for these patients represent real challenges for clinicians.
In France, in 2016, over 1500 patients were treated by Pembrolizumab for NSCLC and its use is still increasing. However, the occurrence of pleural effusions is described as a possible severe adverse reaction in patients with NSCLC treated by Pembrolizumab (2.2% of cases).9 Usually, it is a predominant lymphocytic CD3+ pleural effusion (as in our observation).10
The pleural effusion could also occur during a tumor progression, pseudoprogression or hyperprogression.11 According to the radiological criteria to evaluate the response to immunotherapy, the progression is defined as an increase in tumor burden by ≥25% relative to baseline12 and estimated at 43.5%–69.6% in patients with advanced NSCLC PDL-1 ≥50% treated by Pembrolizumab during the first two years of follow up.13,14 Some patients experience a response after progression, named pseudoprogression and this phenomenon is described in <10% of patients receiving an immunotherapy in the first 12 weeks of treatment. This is the consequence of the recruitment of activated T-lymphocytes to the tumor site induced by the treatment.12 Rapid progression with dramatic acceleration of disease trajectory termed hyperprogression could occur in 4%–29% of patients treated by immunotherapy. Different definitions were suggested for the hyperprogression as a ≥2 increase in the tumor volume at the time of the first evaluation, a time to treatment failure of <2 months, doubling of pace progression or >50% increase in tumor burden in two diameters during immunotherapy.11,12 A tumor biopsy at the time of disease progression could establish the good diagnosis.12 The only one site of the progression in our case was the pleural cavity and the absence of atypical cells in the pleural liquid eliminated these hypotheses.
The third possibility was an infectious etiology. Some cases of tuberculosis were described in patients treated by immunotherapy (incidence 1/1000 per year).15 In the majority of NSCLC cases, it was pulmonary tuberculosis, in men older than 50 years, treated by Nivolumab3,16 and fortuitously discovered during the systematic follow-up by imaging.15 Our case is the first description of pleural tuberculosis occurring during a Pembrolizumab treatment for a patient with NSCLC. The delay of the tuberculosis onset being 3–4 months (like in this case) suggests a reactivation of a latent infection rather than a new infection.3,15 Currently, the role of the PD-1/PD-L-1 pathway in the tuberculosis pathogenesis is not well defined, but appears as a protector against excessive tissue destruction. The exposure to M. tuberculosis increases the expression of PD-1/PD-L-1 on natural killer cells (NK) and T-lymphocytes.16 The activation of this pathway might inhibit the degranulation of CD3+CD8+ cells and the production of interferon gamma (IFNγ), which also limits the NK cell activation, the IFNγ-mediated cell lysis and promotes the Mycobacteria's survival. The administration of an anti-PD-1/PD-L-1 treatment would bring a restoration of these immunological functions.1,3,16 Other mechanisms such as the immunodepression linked to cancer16 or the lymphopenia caused sometimes by immunotherapy1 could also contribute to the occurrence of tuberculosis. Unlike most cases described in literature, the Pembrolizumab was maintained in our case in association with the anti-tuberculosis treatment with a good tolerance and a prolonged therapeutic response.3,16
Faced with the occurrence of a pleural effusion a few weeks after the initiation of an immunotherapy for a NSCLC, clinicians can have difficulties to establish the etiological diagnosis and choose the best therapeutic strategy. Even if the pleural tuberculosis is a rare etiology, it must be recognized and treated in an adapted manner. A screening by a skin test to tuberculin (TST) and IFNγ-release assay (IGRAs) should be considered before starting of immunotherapy in patients with cancer16 in raison of an important tuberculosis-related fatality rate in this population at high-risk for complications (13%).17 There is no gold standard for the diagnosis of latent tuberculosis infection (LTBI)18 and both tests commonly used (TST and IGRAs) have a moderate agreement for its detection in cancer patients.19 Current European guidelines recommend using TST and IGRAs in combination to diagnose LTBI in immunocompromised patients. In practice, the IGRAs should be performed first as TST can boost subsequent IGRA results.20
Currently, there is no consensus on the therapeutic strategy to take concerning the discontinuation or maintenance of immunotherapy during the diagnosis of tuberculosis, so the decision must be taken case by case after discussion in MTOM.
FundingAll the data describes in this case-report have been generated as part of the routine work of the University Hospital of Nancy, France.
Author ContributionsLR and AT drafted the initial manuscript. All authors provided substantial contribution to improve the manuscript in their domain of expertise. All authors approved the final version for submission and accept responsibility for the accuracy and integrity of the work.
Conflict of InterestThe authors declare no conflict of interest.