Since the 6th edition of the tumour, node and metastasis (TNM) classification of lung cancer was published in 2002, the International Association for the Study of Lung Cancer (IASLC) has had the responsibility to revise the subsequent editions of the classification. Based on international databases including patients with lung cancer from around the world and treated with all types of therapies, the IASLC Staging and Prognostic Factors Group (SPFG) proposed changes that were implemented in the 7th, the 8th and the 9th editions of the classification that is conjointly promulgated by the American Joint Committee on Cancer (AJCC) and the Union for International Cancer Control (UICC).1–3
For the 9th edition, 124,581 patients diagnosed with lung cancer between 2011 and 2019 in 75 institutions of 25 countries were registered in the IASLC database. Cancer Research And Biostatistics (CRAB) managed and curated this database that, after exclusions, resulted in a total of 87,043 evaluable patients. Among these, there were 73,197 with non-small cell lung cancer (NSCLC) – 65,060 with clinical stage and 47,513 with pathologic stage –, 8045 with neuroendocrine tumours, 1575 with multiple synchronous primaries, and 4228 with other types of lung cancer. The Bronchogenic Carcinoma Cooperative Group III of the Spanish Society of Pneumology and Thoracic Surgery (SEPAR) contributed 1381 patients, and the University Hospital of Valencia, the University Hospital of Son Espases and the University Clinic of Navarra-CIMA registered 794 additional patients.4 The granularity of this database allowed the analyses of all T, N and M categories, and the proposal for changes in the 9th edition TNM that were accepted by the AJCC5 and the UICC.
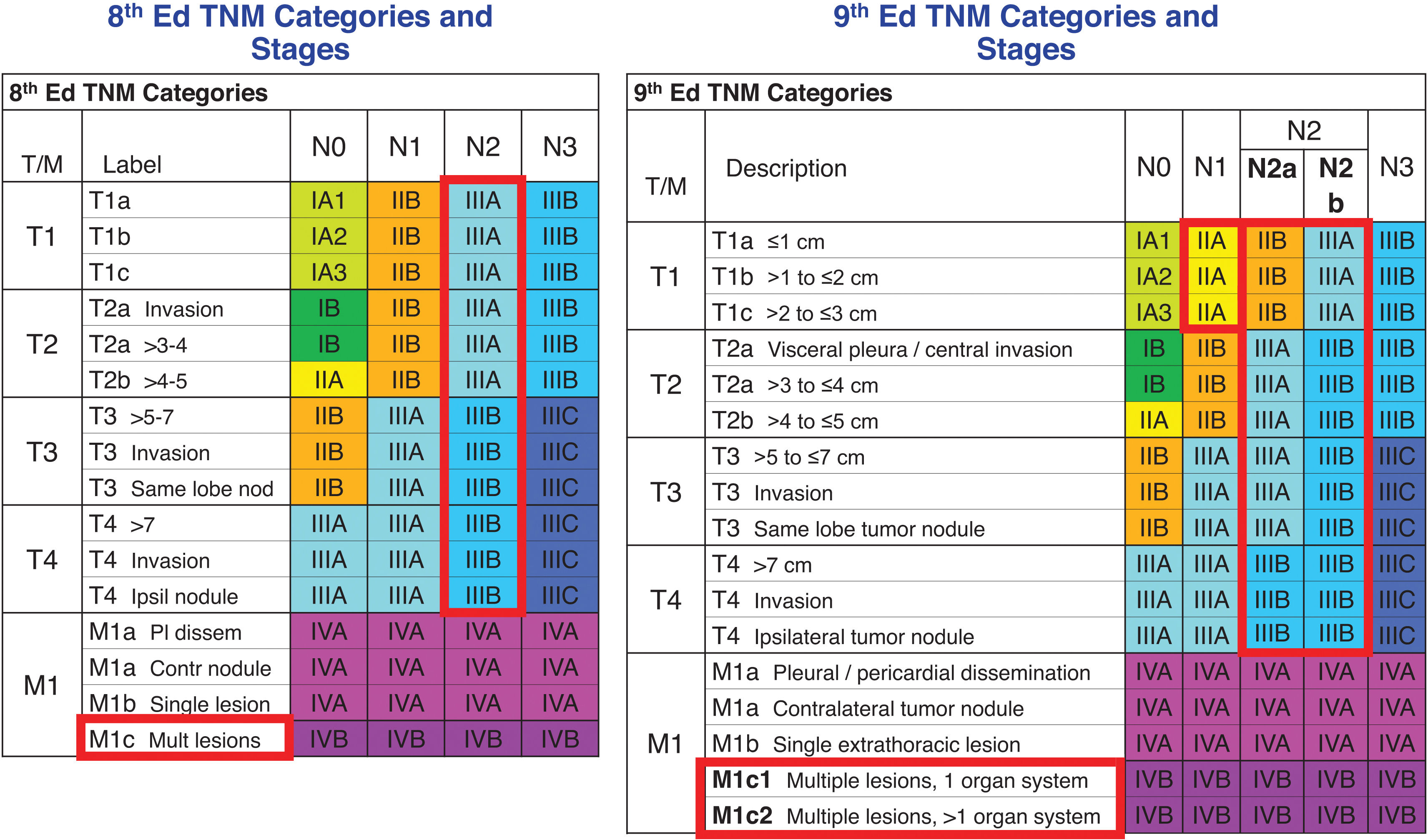
The survival of patients whose primary tumours had been classified with the 8th edition T categories was analysed in the 9th edition database. The 8th edition T categories separated tumours of significantly different prognosis, both at clinical and pathologic evaluation. Therefore, there was no need to modify the 8th edition T categories, which passed unaltered to the 9th edition TNM.6
For decades, there had been discussions on how to classify tumours for which the TNM rules did not fit. To facilitate their homogeneous classification, Mountain had suggested their categories.7 Some of these situations were introduced into the official TNM descriptors but others remained published separately.8 For the 9th edition, these descriptors have been transferred to the official list to make them more visible and facilitate the assignment of their categories. These descriptors are: the invasion of an adjacent lobe is T2a, unless tumour size determines a higher T category; the pericardium, with no distinction between parietal and visceral, the phrenic nerve, the azygos vein, the thoracic nerve roots and the stellate ganglion are T3 descriptors; and the thymus, the vagus nerve, the supra-aortic arteries and brachiocephalic veins, the subclavian vessels, the lamina, the spinal canal, the cervical nerve roots and the brachial plexus are T4. The term Pancoast tumour has been avoided and the structures that classify it as T3 and T4 have been listed, instead.3
There are several ways to quantify nodal disease. In the 7th and the 8th TNM editions, the number of involved nodal zones and the number of involved nodal stations, respectively, separated tumours with different prognosis but only at pathologic evaluation.9,10 Those important findings could not be validated at clinical evaluation and, therefore, were not implemented in their respective TNM editions. In the 9th, a simple way to quantify nodal disease could be validated both at clinical and pathologic evaluations: the subdivision of N2 into N2a – involvement of a single N2 nodal station – and N2b – involvement of several N2 nodal stations. Differences in survival between N2a and N2b held when analysed by T categories, by geographic region, by histologic type, by completeness of resection and in those patients treated with induction therapy. They were also validated with the 8th edition database.11 These findings corroborate the prognostic relevance of the amount of nodal involvement. The possibility to validate them at clinical evaluation is an indication that clinical staging is improving over the years. No doubt the advances in computed tomography and the progressive introduction of positron emission tomography and endobronchial ultrasound transbronchial needle aspiration into clinical practice have improved clinical staging to the point that it is now much closer to the pathologic staging than in the periods of the previous TNM revisions.
Nearly 15,000 patients with distant metastases in the 9th edition database allowed the validation of the 8th edition M1a – intrathoracic metastases – and M1b – single extrathoracic metastasis– categories, and the refinement of M1c – multiple distant extrathoracic metastases. As in the analyses of the 8th edition database, those of the 9th edition confirmed the similar prognosis of M1a and M1b, which remain in the stage IVA. However, in the analyses of the 9th edition database, multiple extrathoracic metastases in a single organ system were found to have better prognosis than multiple extrathoracic metastases in multiple organ systems. These two situations were coded as M1c1 and M1c2, respectively, and remain in stage IVB.12 The term “organ system” was used in the wording of the these descriptors to clarify, for example, that metastases in several bones with no other metastatic sites represent multiple metastases in a single organ system, the musculoskeletal system. In the same way, multiple lymph node involvement beyond the loco-regional lymph nodes included in the lymph node chart represent multiple metastases in the lymphatic system.13 Both situations are classified as M1c1. However, the involvement of the musculoskeletal and the lymphatic systems would be classified as M1c2.
The subdivision of N2 into N2a and N2b created new tumour groups that did not explicitly exist in the previous TNM editions. Survival of these new tumours was analysed and, depending on their survival, they were assigned to either the same or to different stages compared with the 8th edition stages. The changes in the 9th edition are: T1N2a was assigned to stage IIB; T2N2b was assigned to stage IIIB; and T3N2a was assigned to stage IIIA. Additionally, T1N1 tumours were found to have better prognosis that those in the 8th edition stage IIB – the stage where they belonged –, and were downstaged to the 9th edition stage IIA. These changes held in all cell types, in all geographic regions, in different study periods, in patient with different performance status, and in the different types of data sets.3Fig. 1 shows the 9th edition stage groups compared with those of the 8th edition.
Stage groups of the 8th and of the 9th editions of the TNM classification for lung cancer. Cells highlighted in red are those where changes have taken place. Nod: nodules; Ipsi: ipsilateral; Pl: pleural; dissem: dissemination; Contr: contralateral; Mult: multiple.
Taken from Ref.3with permission.
Cancer cells spread through air spaces (STAS) beyond the tumour edge can be found in over 20% of stage I lung cancers. STAS has been proven to be a strong prognostic factor in all cell types and all types of lung resections. In the 9th edition TNM it will be added to lymphovascular invasion, perineural permeation and visceral pleura invasion as a pathologic descriptor.14
The innovations of the 9th edition TNM improve our understanding of the prognostic impact of the anatomic extent of lung cancer but require a thorough clinical and pathologic evaluation of mediastinal nodal disease and of the number and organ location of the distant metastases. Only with a sound clinical and pathologic staging will the innovations be useful in clinical practice and research.
Contribution of Each AuthorRRP: Conceptualization, writing, revising and editing.
HA: Conceptualization, revising and editing.
VWR: Conceptualization, revising and editing.
Conflict of InterestRamón Rami-Porta has no conflicts of interest. Hisao Asamura declares lecture fees from Covidien Japan and Johnson & Johnson. Valerie W. Rusch declares her work is supported in part by the NIH Cancer Center Support Grant P30-CA008748 to Memorial Sloan Kettering Cancer Center, New York, NY, USA; participation in the Data Safety Monitoring Board for UK MARS-2 trial (completed and published); and being Chair, IASLC Staging and Prognostic Factors Group (2024–2031).