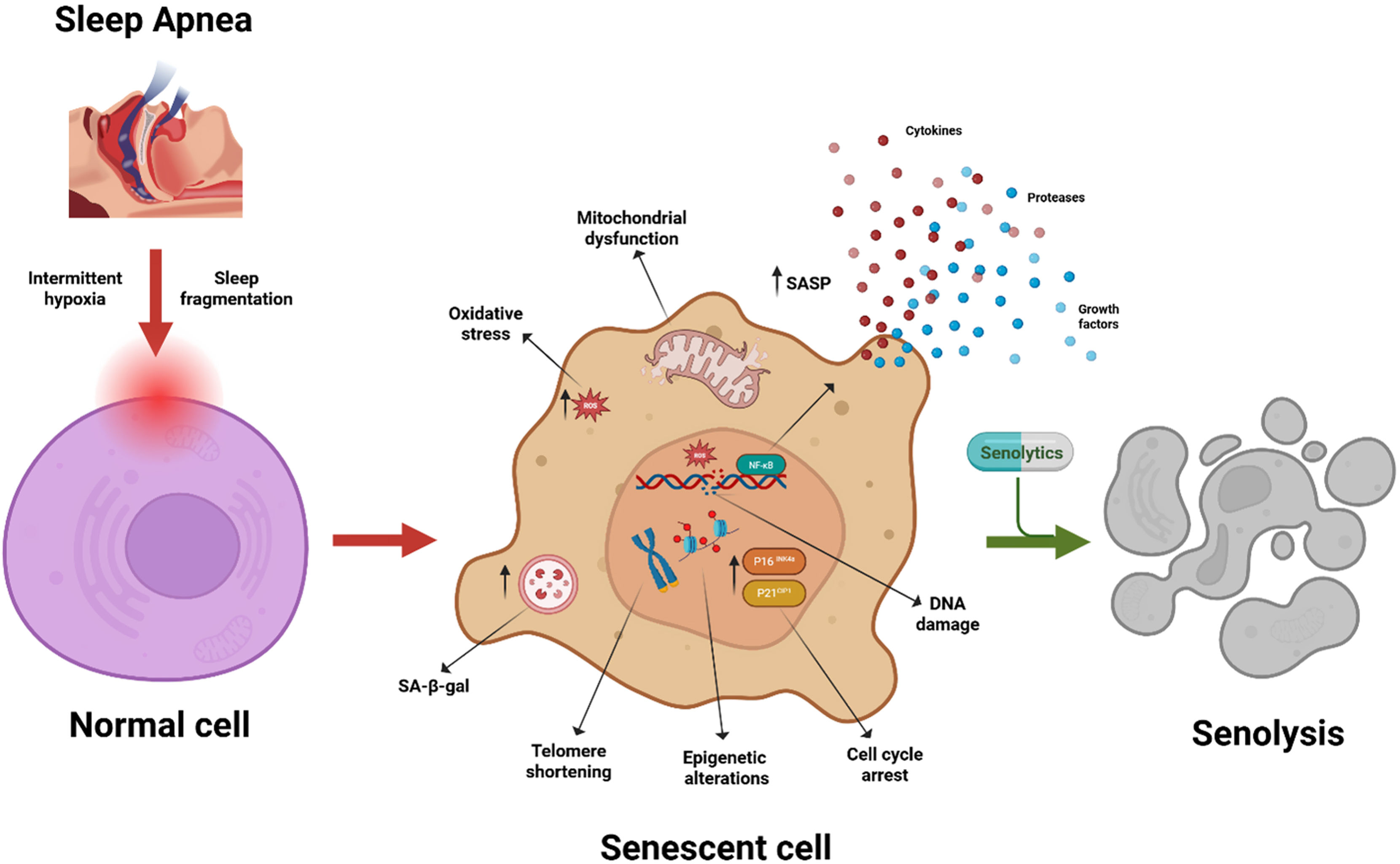
Obstructive sleep apnea (OSA) is characterized by recurrent upper airway collapse during sleep, resulting in intermittent hypoxia (IH), episodic hypercapnia, sleep fragmentation, and swings in intrathoracic pressures. OSA has been traditionally viewed as a contributor to daytime somnolence, cardiovascular and metabolic risk, but emerging data place OSA squarely within the biology of aging. More specifically, the chronic low-grade inflammation of OSA activates cellular pathways that underlie accelerated biological aging and senescence. Reframing OSA in this light not only provides a unified conceptual framework for its diverse comorbidities, but also suggests novel, “senotherapy”-based interventions aimed at mitigating end-organ damage and improving long-term outcomes.
Biological aging encompasses the cumulative decline in cellular, tissue, and organ functions, distinguishing between true “physiological/biological age” and mere chronological age. Central to this process is cellular senescence (Fig. 1), a state of irreversible cell-cycle arrest triggered by telomere attrition, DNA damage, oxidative stress, oncogenic signaling, or mitochondrial dysfunction.1 Once the process of senescence is initiated, senescent cells secrete a complex and heterogeneous “cocktail” of inflammatory cytokines, chemokines, growth factors, and proteases – the senescence-associated secretory phenotype (SASP) – which alters the tissue microenvironment, promotes fibrosis, and dysregulates immune surveillance.1 Although senescence prevents proliferation of damaged cells, persistent or excessive accumulation of senescent cells within a tissue drives chronic inflammation (“inflammaging”) and impairs tissue repair, linking aging with age-related diseases.1 Senescence is characterized by biomarkers including upregulation of the major transcription factors underlying its initiation, p16INK4a and p21CIP1, increased senescence-associated β-galactosidase activity, DNA damage foci, telomere attrition and shortening, and SASP profiles. Epigenetic clocks—particularly DNAmPhenoAge and GrimAge—derive biological age from DNA methylation patterns and strongly predict morbidity, mortality, and functional decline.2 Age acceleration, defined as the difference between epigenetic and chronological age, quantifies how disease or exposure hastens the aging trajectory toward disease and death.
Persistent chronic pathophysiological stressors, such as chronic obstructive pulmonary disease (COPD), diabetes mellitus, and cardiovascular disease (CVD), are all characterized by accelerated biological aging processes that promote sustained cellular dysfunction and inflammation. In COPD, increased senescent epithelial and immune cells, elevated oxidative DNA damage, and telomere shortening contribute to progressive airflow limitation and comorbidities.3 Diabetic tissues accumulate advanced glycation end-products and mitochondrial reactive oxygen species (ROS), inducing epigenetic shifts that mirror aged phenotypes and impair tissue repair.4 Similarly, CVD is marked by senescent vascular smooth muscle cells and endothelial cells, SASP-driven inflammation, and extracellular matrix remodeling, all of which exacerbate vascular stiffness and myocardial dysfunction.5 These accelerated aging signatures reflect chronic disease progression as both a driver and outcome of senescence-driven organ decline. Thus, the larger the biological age-chronological age difference, the earlier the decompensation and ultimately death of the individual.
The unique patterns of IH and sleep disruption in OSA individually and collectively exert a potent and multifaceted drive toward accelerated biological aging. Leukocyte telomere attrition in moderate-to-severe OSA patients is significantly greater than in age-matched controls and correlates directly with apnea–hypopnea index and nocturnal desaturation burden, indicating enhanced replicative senescence and genomic instability.6 DNA methylation-based clocks—such as Horvath and PhenoAge clocks—provide an estimate of biological age. Recent epigenome-wide study by Cortese and colleagues shows that individuals with OSA exhibit accelerated epigenetic aging, independent of traditional risk factors. Importantly, the study also demonstrated that adherent CPAP therapy partially reverses this epigenetic age acceleration: patients using CPAP for at least four hours per night over one year exhibited an average reduction of ∼3 years in DNAm-derived biological age compared to baseline.7 At the tissue level, chronic IH elevates expression of cell-cycle inhibitors p16INK4a in endothelial and adipose compartments, accompanied by increased SA-β-galactosidase activity and secretion of SASP cytokines such as IL-6, thereby fostering a pro-inflammatory microenvironment and endothelial and metabolic dysfunction.8 Systemically, a meta-analysis shows that OSA elevates systemic inflammatory mediators characteristic of the SASP, such as IL-6, TNF-α, and ICAM-1, further linking OSA with inflammaging and cardiometabolic dysregulation.9 Together, these findings underscore OSA as a robust promoter of age acceleration across molecular, cellular, and systemic domains.
The IH cycles of OSA mimic ischemia–reperfusion injury, rapidly generating ROS that damage nuclear and mitochondrial DNA. ROS activate p53 and NF-κB transcriptional programs, upregulating senescence genes and SASP mediators.10 Pinilla et al. demonstrated that IH impairs mitochondrial dynamics—reducing mitophagy and increasing mitochondrial DNA deletions—in adipose tissue, fueling systemic metabolic dysfunction.11 Concurrently, sleep fragmentation disrupts circadian oscillators (CLOCK-BMAL1), which govern DNA repair and antioxidant enzyme expression.12 Prolonged sleep fragmentation results in increased expression of p16INK4a in the vasculature and is associated with progressive vascular dysfunction.13 It also exacerbates oxidative damage and blunts stress responses, synergizing with IH to accelerate cellular aging. OSA also alters the gut microbiome, decreasing beneficial short-chain fatty acid producers and increasing endotoxin-bearing taxa, which remarkably resemble the changes occurring in the aging microbiome.
Continuous positive airway pressure (CPAP) therapy remains the gold standard for OSA, effectively reversing hypoxia and improving daytime symptoms. However, the ability of adherent CPAP treatment to reverse established cellular senescence and epigenetic age acceleration is limited. CPAP therapy reduces circulating inflammatory markers, but telomere length reduction and epigenetic age often remain unchanged even after months of CPAP adherence.7 Senolytic therapies – small molecules that selectively eliminate senescent cells – offer a promising adjunctive therapeutic option. Badran et al. recently demonstrated using a pre-clinical model of IH mimicking OSA that senolytic-facilitated clearance of senescent cells led to markedly improved reversal multi-end-organ dysfunction when compared to adherent “CPAP” treatment.8 Indeed, when compared to standard “CPAP” alone, concurrent administration of a senolytic compound significantly improved many of the morbidity domains affected by OSA, namely cognitive function, sleep propensity, coronary artery dilation, reduced myocardial fibrosis, insulin resistance, and restored vascular reactivity.8 Zhu et al. discovered that dasatinib plus quercetin reduces senescent cell burden, lowers SASP secretion, and restores endothelial function in aged mice,14 and this combinatorial drug treatment may be useful in OSA. Similarly, senomorphics – agents that suppress SASP without killing cells – such as rapamycin analogs, may attenuate inflammation and improve mitochondrial health.15 Integrating CPAP with intermittent senotherapy regimens could therefore more fully and comprehensively address both upstream drivers (IH, sleep fragmentation) and downstream aging/senescence mechanisms. Future clinical trials should incorporate age acceleration metrics—telomere length, DNAmPhenoAge, GrimAge—and SASP panels as endpoints to evaluate therapeutic efficacy beyond traditional apnea indices.
Translating senescence-targeted therapies in OSA warrants rigorous trial design. Key considerations include optimal timing (early disease vs. advanced comorbidity), dosing schedules (pulsatile vs. continuous), and safety in a population often manifesting a high disease burden. Patient stratification by baseline age acceleration or senescence biomarker profiles may personalize interventions. Novel imaging tracers for senescence markers and liquid biopsy assays for senescent cell–derived extracellular vesicles could enable real-time monitoring of treatment response. Furthermore, multi-omic analyses—integrating transcriptomics, proteomics, and metabolomics—in well-characterized OSA cohorts will deepen understanding of aging pathways and reveal new therapeutic targets. Investigating interactions between OSA, aging, and coexisting conditions (COPD, diabetes, Alzheimer's disease) may identify common nodes for intervention and guide comprehensive approaches to improve health span.
In summary, OSA is not merely a disorder of disrupted sleep and gas exchange; it is a potent accelerator of biological aging, driving cellular senescence, chronic inflammation, and end-organ dysfunction. Recognizing OSA within the framework of aging biology unifies its link to chronic disease and mortality and opens novel mechanistic and therapeutic avenues. Reframing OSA as an aging disorder not only deepens mechanistic understanding but may also stimulate novel interventions aimed at extending health span and lifespan.
Financial SupportMB and DG are currently supported in part by NIH grant HL166617. DG also receives partial support from NIH grants HL169266 and P20GM103434 to the West Virginia IDeA Network of Biomedical Research Excellence.
Conflict of InterestThe authors have no financial or other conflict of interest to declare.