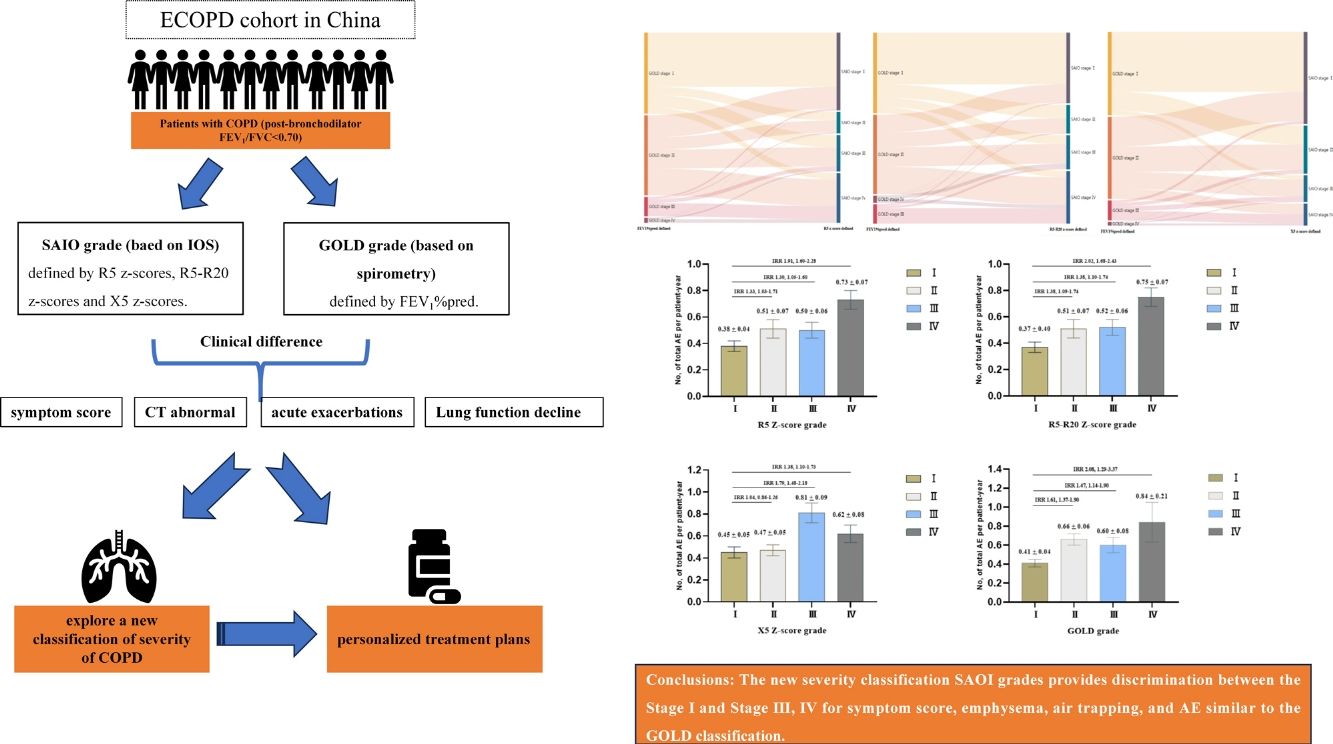
Recently, the severities of chronic obstructive pulmonary disease (COPD) can also be assessed by impulse oscillometry (IOS). This study aimed to explore a new classification of severity of COPD based on IOS and associations with acute exacerbations (AE) in patients with COPD.
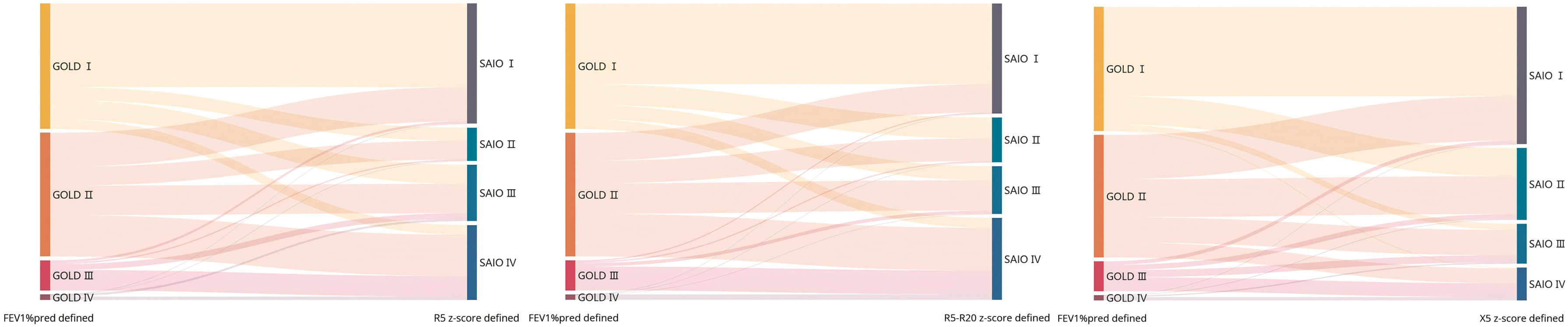
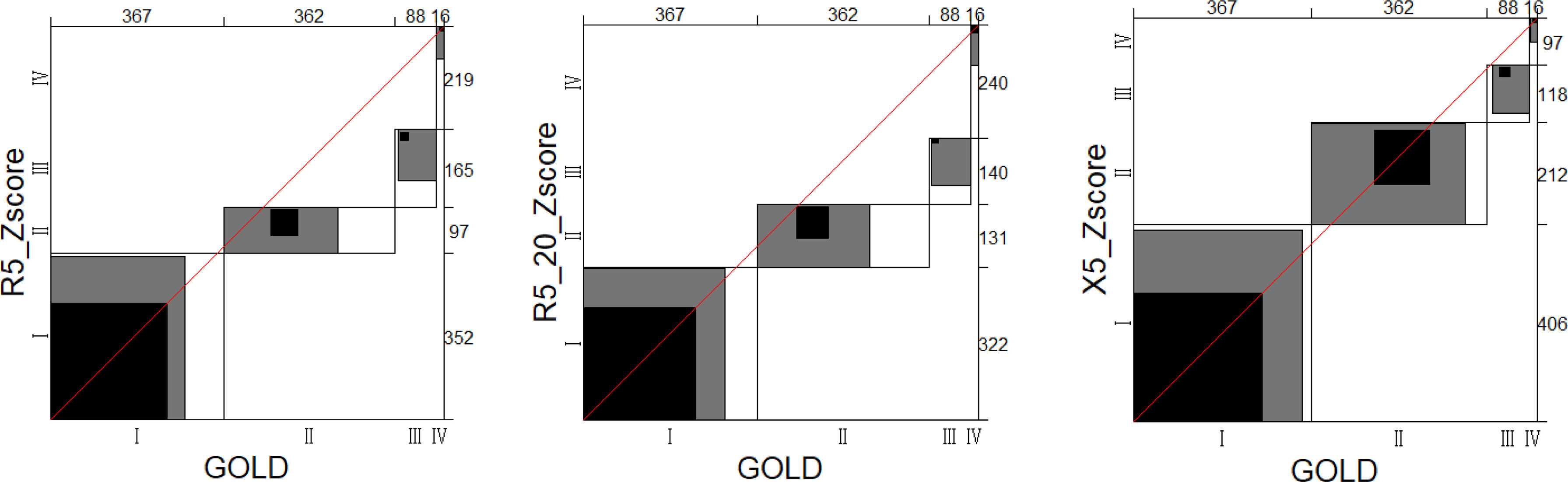
MethodsThe data of our study were based on the baseline and 2-year follow-up data of a prospective cohort in China. COPD was defined as post-bronchodilator FEV1/FVC <0.70. A new severity classification (staging of airflow obstruction by IOS, SAIO) was evaluated based on IOS parameters (R5, R5–R20, and X5 z-scores). We quantified using the weighted Bangdiwala B for agreement of severities of COPD between IOS parameters and FEV1%pred. The differences among SAIO stages were performed in symptom scores and imaging using analysis of covariance, and in the AE using Poisson regression.
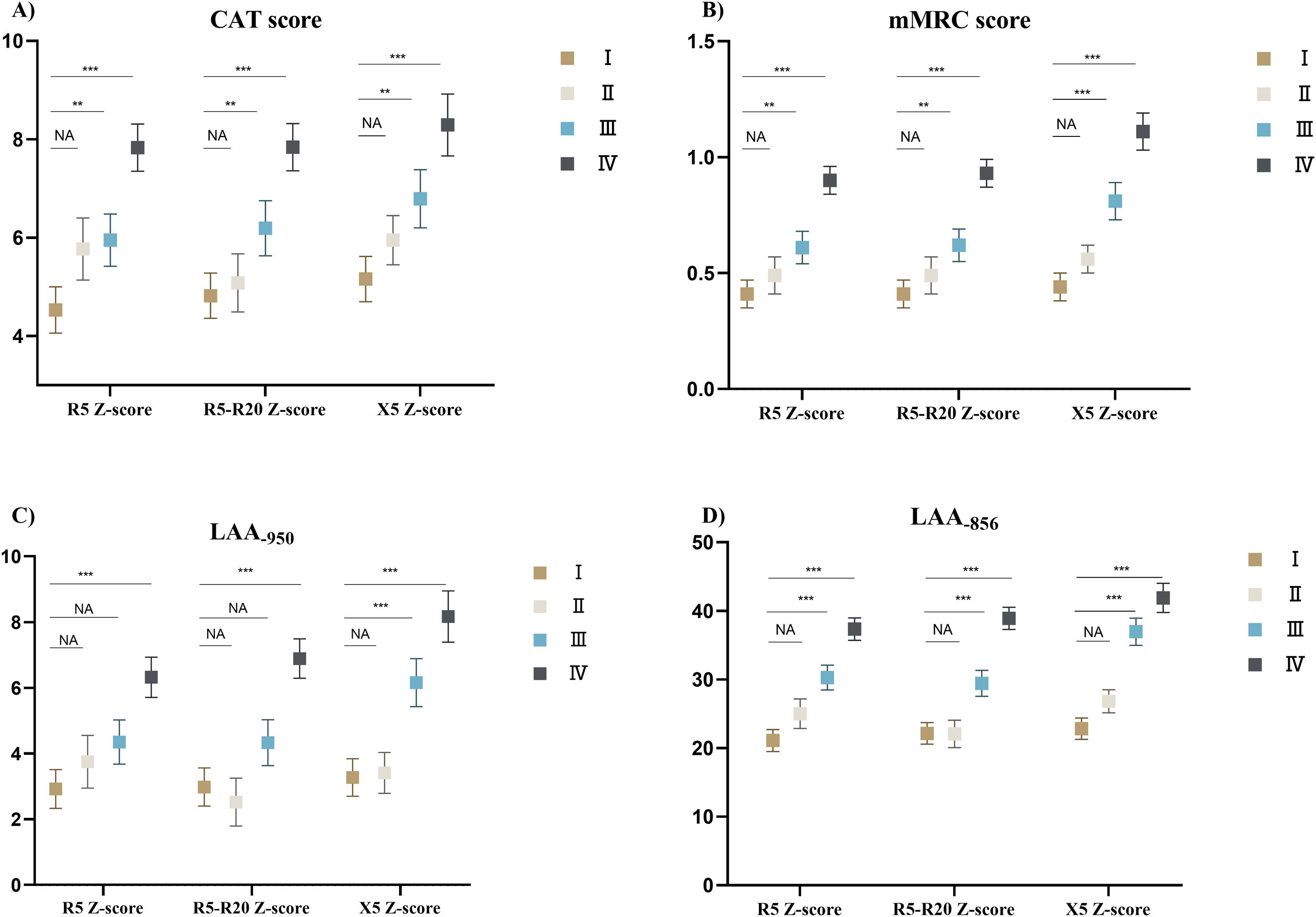
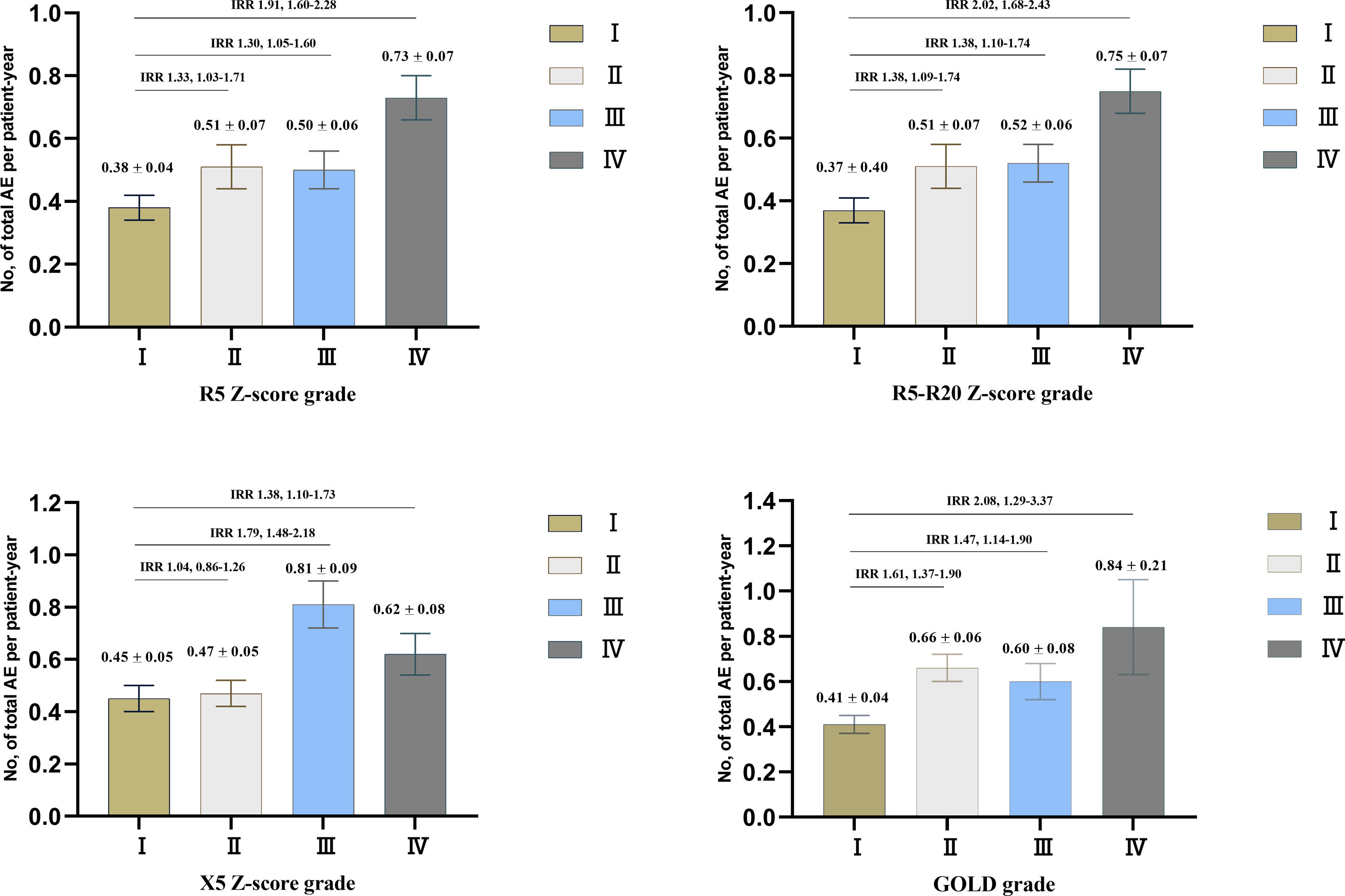
ResultsOverall, 833 patients with COPD were included in this study. The weighted Bangdiwala B of R5, R5–R20, X5 z-scores, and FEV1%pred for evaluating agreement of the severities of COPD was 0.68, 0.70 and 0.83, respectively. The SAIO classifications system identified a greater number of patients with stage III–IV. SAIO provided significant discrimination between the stage I and stage III, IV for symptom scores, emphysema, and air trapping. SAIO provided significant discrimination between the stage I and other stages for AE.
ConclusionsThe SAIO classifications provide discrimination between the stage I and stage III, IV for symptom scores, emphysema, air trapping, and AE, similar to the GOLD classifications.
Trial registrationChinese Clinical Trial Registry, ChiCTR1900024643. Registered on 19 July, 2019.