Hemoptysis is a common symptom in pulmonology, accounting for up to 10% of consultations and 15% of admissions in some series [1]. It refers to bleeding from the subglottic airways and varies in severity from mild hemoptoic sputum – seen in up to 90% of cases – to massive hemoptysis, which carries a mortality risk exceeding 50% [2,3]. Regardless of severity, it constitutes a medical emergency requiring hospitalization and potentially specialized care. Bronchial arterial embolization (BAE) remains the standard treatment, with bleeding control rates between 70% and 99% [3]. However, this technique depends on immediate access to interventional radiology, which is unavailable in many hospitals. Consequently, frontline clinicians – such as pulmonologists, intensivists, and emergency physicians – have sought accessible alternatives to manage hemoptysis or bridge patients until embolization is feasible. Tranexamic acid (TXA), a synthetic antifibrinolytic derived from Epsilon-Amino-Caproic Acid, inhibits plasminogen activation and delays fibrinolysis [4]. Known for its efficacy in trauma-related bleeding, it is widely used via intravenous routes in emergency settings. More recently, TXA has been administered via inhalation to treat hemoptysis, aiming to deliver local hemostasis with fewer systemic side effects. While promising, this off-label practice is supported mainly by case series and limited trials. Hankerson et al. [5] first reported inhaled TXA use in 2015 for palliative management of low-volume hemoptysis. In 2018, Wand et al. [6] conducted a double-blind RCT demonstrating cessation of hemoptysis within five days in 96% of patients treated, compared to 50% in the placebo group. Gopinath et al. [7] later compared inhaled and intravenous TXA, reporting reduced bleeding, fewer embolizations, and shorter hospital stays in the inhaled group. Additional case series have echoed these findings, but the evidence remains sparse and heterogeneous [8–15]. Given the limited but encouraging data, we conducted a retrospective, bi-centric study with a larger sample to assess the effectiveness of nebulized TXA (NebTXA) in patients hospitalized for mild to moderate hemoptysis. Our primary aim was to characterize patients responding to NebTXA, and secondarily, to compare them with those who ultimately required embolization.
We conducted a retrospective, observational, bi-centered study at the Aix-Pertuis Intercommunal Hospital Center and the Marseille North University Hospital (France) between 2020 and 2023. Ethical approval was granted via the Health Data Access Portal (PADS: GK25MX); patient data were anonymized. Included were adult patients hospitalized for mild to moderate hemoptysis (<200mL/24h or <100mL in a single episode), excluding those with severe or unstable conditions, TXA use before admission, or contraindications. Hemoptysis severity and exclusion criteria were defined by clinical and hemodynamic thresholds, including oxygen or vasopressor need, respiratory rate ≥30, or mechanical ventilation. Patients were identified through ICD-10 code R04.2 and had received nebulized tranexamic acid (NebTXA) 500mg TID via nebulization over ≥15min. Collected data included demographics, underlying diseases (airway, vascular, malignant, iatrogenic), clinical and imaging findings, hemoptysis characteristics, and outcomes.
The primary objective was to determine the clinical profile of patients in whom NebTXA successfully controlled bleeding without requiring invasive management (interventional bronchoscopy, embolization, or surgery). Secondary objectives included evaluating time to hemoptysis resolution, hospital length of stay, need for embolization after treatment failure, and overall, in-hospital mortality. We also compared clinical characteristics between responders and non-responders (embolized patients precisely) to identify predictors of treatment failure. Data was analyzed using RStudio (2022.07.2). Categorical variables were compared using χ2 or Fisher's exact test, continuous variables by t-test or Mann–Whitney U. Logistic regression (univariate and multivariate) identified predictors of NebTXA failure, with significance set at p<0.05.
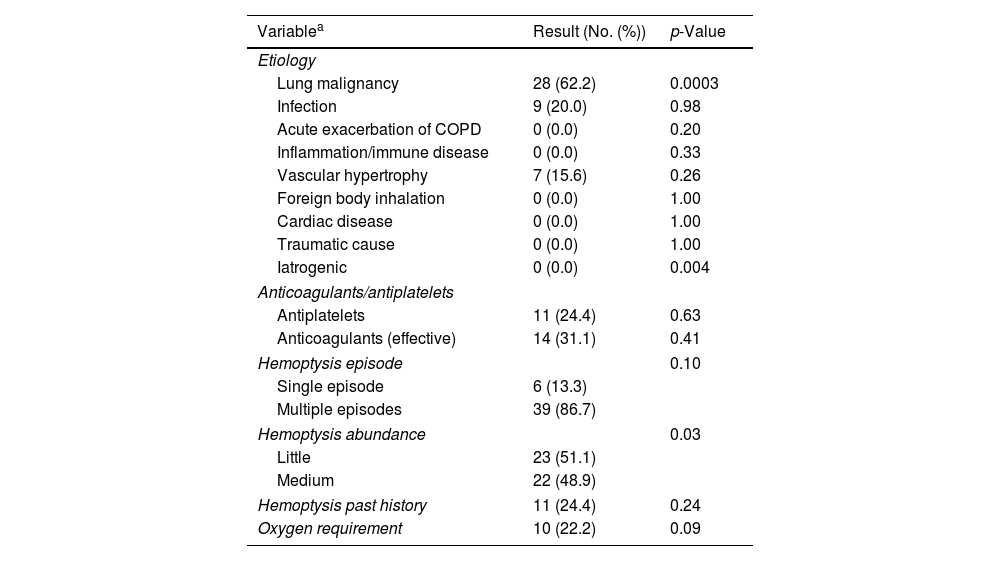
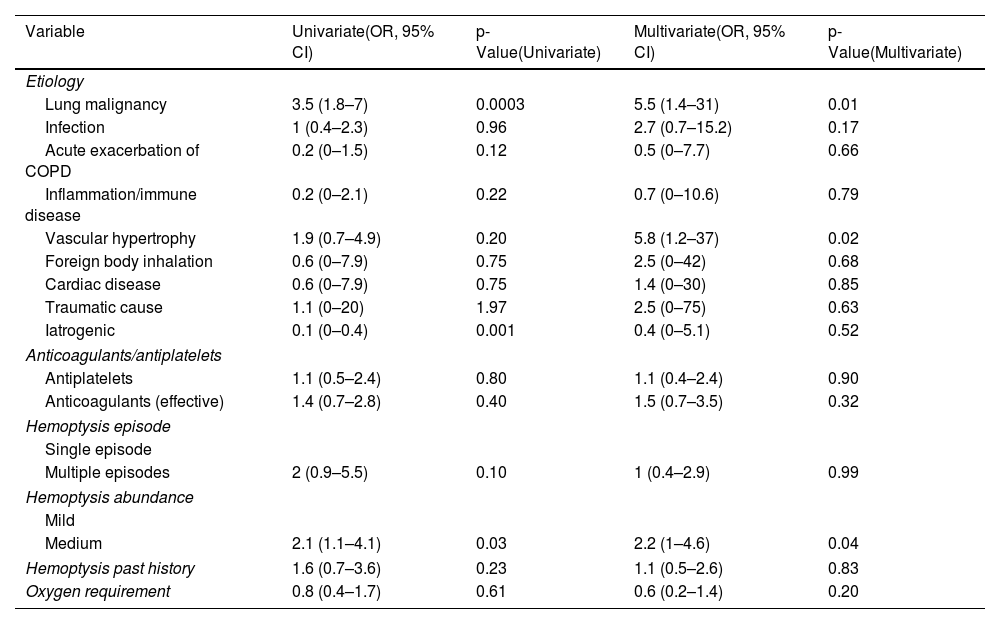
Between January 2020 and December 2023, 189 patients met inclusion criteria (62 at Aix-Pertuis, 127 at Marseille North). Most were male (69.8%), median age 63 years (IQR 52–73), and heavy smokers (52.9%). Comorbidities included cardiovascular diseases (43.4%), COPD/emphysema (15.3%), and bronchiectasis (4.2%). At admission, 25.4% were on antiplatelet therapy and 26.5% on curative anticoagulation. Hemoptysis was recent (<7 days in 77.3%), recurrent (77.8%), low volume (<100mL in 69.8%), and required oxygen in 25.4% of cases. A thoracic angio-CT was performed in 88.9%, revealing consolidations or ground-glass opacities in 47.6% and an embolizable vascular target in 40.8% (92.2% bronchial origin). Main etiologies were bronchopulmonary cancers (39.2%, with 52.7% inaugural), infections (27.5%), and iatrogenic causes (12.7%). Median hospital stay was 7 days (IQR 5–12). Nineteen patients died (10.1%), mostly from cancer progression or hospital-acquired infections; none were attributed to NebTXA. NebTXA achieved hemoptysis cessation within 5 days in 162 patients (85.7%), including 55 with cancer. Treatment failure (27 patients) was more frequent in those with cancer (70.4%), recurrent hemoptysis, and oxygen need. These patients also had longer stays (median 12 vs. 7 days). Bronchoscopy, embolization, and surgery were performed in 45.5%, 23.8%, and 1.1% of patients, respectively. Embolization targeted bronchial arteries in 93.3% and was more common in moderate hemoptysis (48.9%, p=0.03). As you can see in Table 1, a predominance of bronchopulmonary cancers (62.2%, p=0.0003) was observed in embolized patients. Conversely, iatrogenic hemoptysis has never been embolized (0%, p=0.004). An embolization procedure was performed more often and significantly in patients with moderately abundant hemoptysis (48.9% of patients) (p=0.03). Mortality was lower (6.7%) although the difference with the non-embolized population was not significant (p=0.571). Multivariate analysis (Table 2) confirmed bronchial cancer and moderate hemoptysis as predictors of NebTXA failure. Vascular anomalies and idiopathic causes were also associated with treatment failure. Antithrombotic therapy did not affect outcomes or mortality, which was higher in cancer patients with moderate hemoptysis (57.9%, p=0.0094) and oxygen dependence (57.9%, p=0.0009).
Variables for patients undergoing embolization (multivariate analysis).
| Variablea | Result (No. (%)) | p-Value |
|---|---|---|
| Etiology | ||
| Lung malignancy | 28 (62.2) | 0.0003 |
| Infection | 9 (20.0) | 0.98 |
| Acute exacerbation of COPD | 0 (0.0) | 0.20 |
| Inflammation/immune disease | 0 (0.0) | 0.33 |
| Vascular hypertrophy | 7 (15.6) | 0.26 |
| Foreign body inhalation | 0 (0.0) | 1.00 |
| Cardiac disease | 0 (0.0) | 1.00 |
| Traumatic cause | 0 (0.0) | 1.00 |
| Iatrogenic | 0 (0.0) | 0.004 |
| Anticoagulants/antiplatelets | ||
| Antiplatelets | 11 (24.4) | 0.63 |
| Anticoagulants (effective) | 14 (31.1) | 0.41 |
| Hemoptysis episode | 0.10 | |
| Single episode | 6 (13.3) | |
| Multiple episodes | 39 (86.7) | |
| Hemoptysis abundance | 0.03 | |
| Little | 23 (51.1) | |
| Medium | 22 (48.9) | |
| Hemoptysis past history | 11 (24.4) | 0.24 |
| Oxygen requirement | 10 (22.2) | 0.09 |
Variables analyzed in case of NebTXA failure (univariate and multivariate analysis) (CI: 95%).
| Variable | Univariate(OR, 95% CI) | p-Value(Univariate) | Multivariate(OR, 95% CI) | p-Value(Multivariate) |
|---|---|---|---|---|
| Etiology | ||||
| Lung malignancy | 3.5 (1.8–7) | 0.0003 | 5.5 (1.4–31) | 0.01 |
| Infection | 1 (0.4–2.3) | 0.96 | 2.7 (0.7–15.2) | 0.17 |
| Acute exacerbation of COPD | 0.2 (0–1.5) | 0.12 | 0.5 (0–7.7) | 0.66 |
| Inflammation/immune disease | 0.2 (0–2.1) | 0.22 | 0.7 (0–10.6) | 0.79 |
| Vascular hypertrophy | 1.9 (0.7–4.9) | 0.20 | 5.8 (1.2–37) | 0.02 |
| Foreign body inhalation | 0.6 (0–7.9) | 0.75 | 2.5 (0–42) | 0.68 |
| Cardiac disease | 0.6 (0–7.9) | 0.75 | 1.4 (0–30) | 0.85 |
| Traumatic cause | 1.1 (0–20) | 1.97 | 2.5 (0–75) | 0.63 |
| Iatrogenic | 0.1 (0–0.4) | 0.001 | 0.4 (0–5.1) | 0.52 |
| Anticoagulants/antiplatelets | ||||
| Antiplatelets | 1.1 (0.5–2.4) | 0.80 | 1.1 (0.4–2.4) | 0.90 |
| Anticoagulants (effective) | 1.4 (0.7–2.8) | 0.40 | 1.5 (0.7–3.5) | 0.32 |
| Hemoptysis episode | ||||
| Single episode | ||||
| Multiple episodes | 2 (0.9–5.5) | 0.10 | 1 (0.4–2.9) | 0.99 |
| Hemoptysis abundance | ||||
| Mild | ||||
| Medium | 2.1 (1.1–4.1) | 0.03 | 2.2 (1–4.6) | 0.04 |
| Hemoptysis past history | 1.6 (0.7–3.6) | 0.23 | 1.1 (0.5–2.6) | 0.83 |
| Oxygen requirement | 0.8 (0.4–1.7) | 0.61 | 0.6 (0.2–1.4) | 0.20 |
Our cohort of 189 patients, mostly male smokers with cardiovascular comorbidities, reflects real-life presentations of hemoptysis. Nebulized tranexamic acid (NebTXA) appeared effective for mild to moderate bleeding, particularly in single-episode cases without oxygen dependence. Though not all findings reached significance, favorable trends were observed in infections, inflammatory diseases, and iatrogenic causes. This aligns with prior studies. Zhang et al. [16] confirmed the safety and local efficacy of inhaled TXA, while Alkazemi et al. [15] and Mahalingam et al. [17] supported its role in reducing procedures and hospital stay in non-massive hemoptysis. Our protocol (500mg/5mL, 3–4 times/day) matches these standards, reinforcing external validity. Antiplatelet and anticoagulant use did not affect outcomes, though in temporary discontinuation caution remains warranted. Shorter hospital stays suggest early symptom control with NebTXA, reflecting it may reduce the need for invasive procedures. Multivariate analysis identified bronchopulmonary cancer and moderate-volume bleeding as independent predictors of treatment failure and need for bronchial artery embolization (BAE). Patients undergoing bronchial artery embolization had more frequent, moderate-volume hemoptysis, fewer radiologic ground-glass opacities, and lower mortality. Most deaths were due to malignancy progression or complications, not NebTXA itself, reinforcing its safety. Compared to Wand et al. [6], our cohort included fewer patients with bronchiectasis, likely due to local referral patterns. NebTXA appeared beneficial in reversible etiologies, but spontaneous resolution may account for part of its effect. Our findings help identify most likely ideal candidates and high-risk profiles. Strengths include largest to date sample size and multivariate adjustments. Limitations involve retrospective design, limited power preventing initial primary objective, potential confounding, and local recruitment bias. Prospective studies are warranted to define NebTXA's place, especially in oncology and resource-limited settings.
This study describes a diverse real-life cohort of 189 patients and suggests that NebTXA may be relevant for managing low- to moderate-abundance hemoptysis, particularly in first episodes without oxygen therapy and involving reversible etiologies. However, this remains an exploratory analysis of a poorly standardized practice. In cases such as bronchopulmonary neoplasia, its use should not replace consultation with interventional radiologists. These findings support future prospective studies comparing NebTXA to standard care, especially in centers lacking interventional capabilities.
CRediT authorship contribution statement- -
Virgile Caban: Conceptualization, data collection, analysis, manuscript drafting.
- -
Julie Tronchetti: Methodology, data interpretation, data review, manuscript review.
- -
Jean-Baptiste Lovato: Methodology, data collection, data interpretation.
- -
Stéphanie Martinez: Methodology, data collection, data review.
- -
Hervé Dutau: Supervision, critical revision of the manuscript.
- -
Philippe Astoul: Supervision, methodology, project administration, manuscript review.
Artificial intelligence tools were used to assist in editing and formatting but not in data analysis or content generation.
FundingThis study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflicts of interestThe authors declare no conflicts of interest.