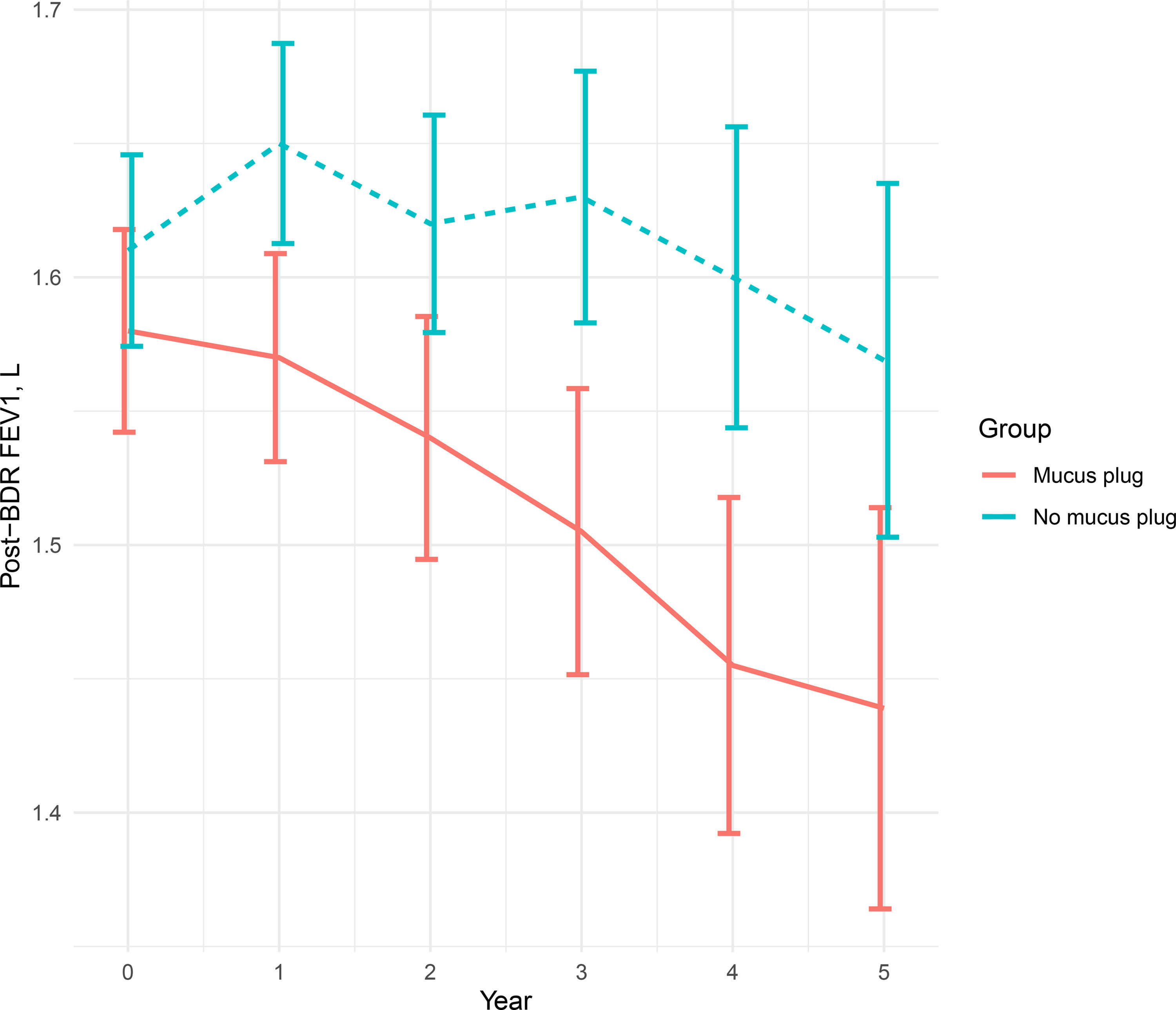
Mucus plugs identified through chest computed tomography (CT) scans have emerged as potential prognostic factors in chronic obstructive pulmonary disease (COPD). This 5-year longitudinal study investigated their impact on exacerbations and FEV1 decline.
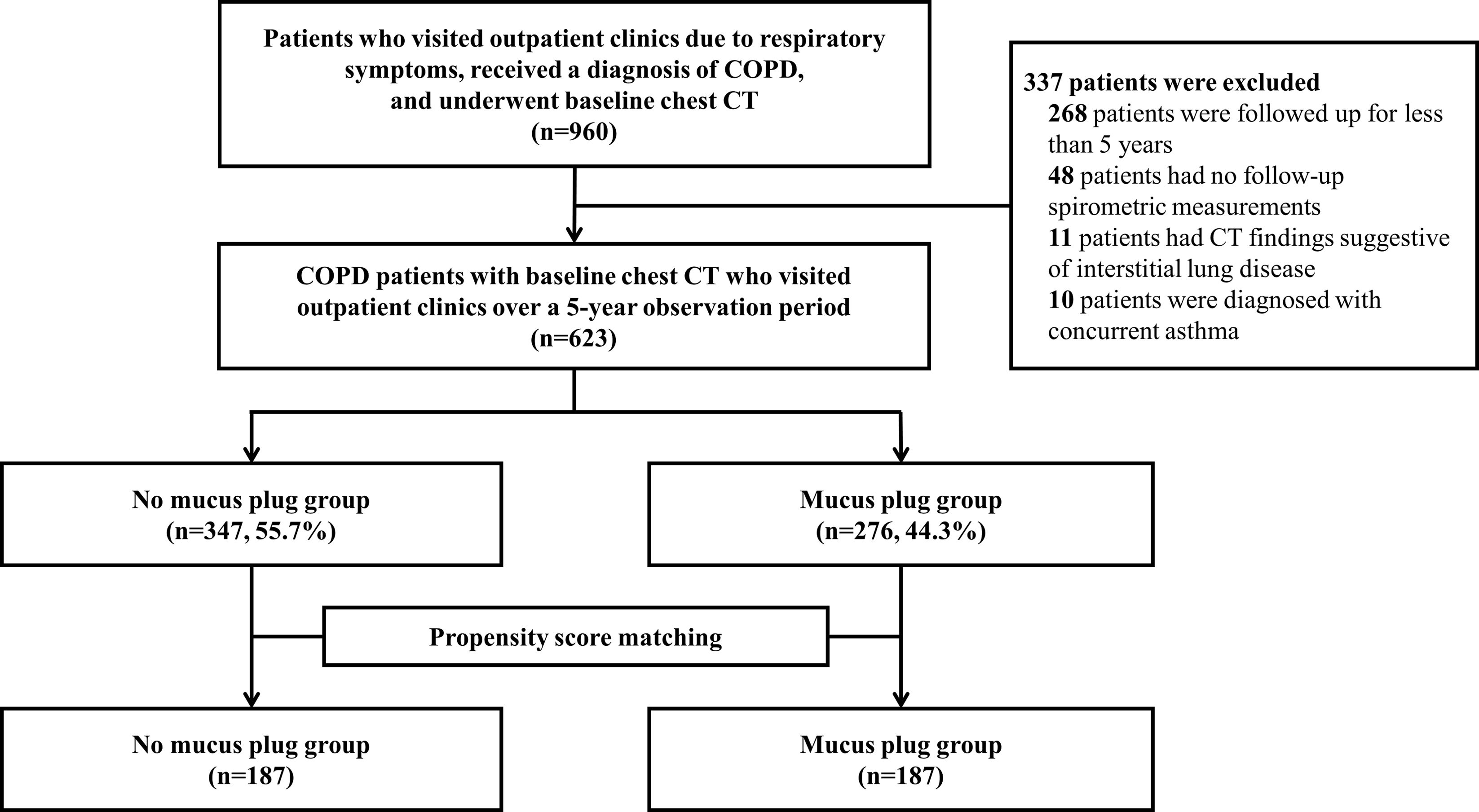
MethodsCOPD patients with baseline chest CT and spirometric assessments were categorized based on mucus plug presence. Propensity-score matching yielded balanced groups. Exacerbation rates, time to exacerbation events, hazard ratio (HR) for exacerbations, and annual rates of FEV1 decline were evaluated. Sensitivity analysis was performed with stratification according to mucus plug scores of 0, 1–2, and ≥3.
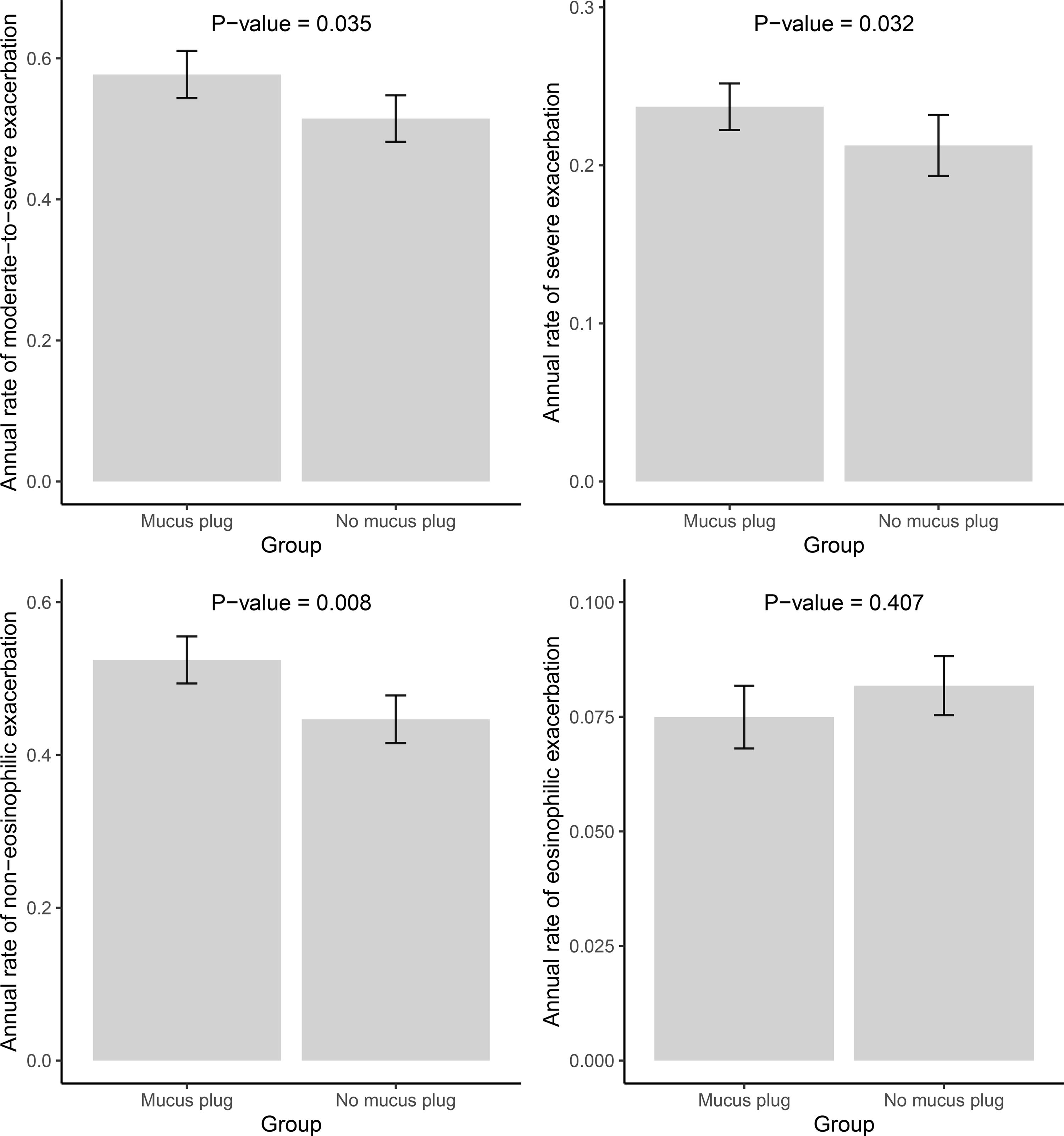
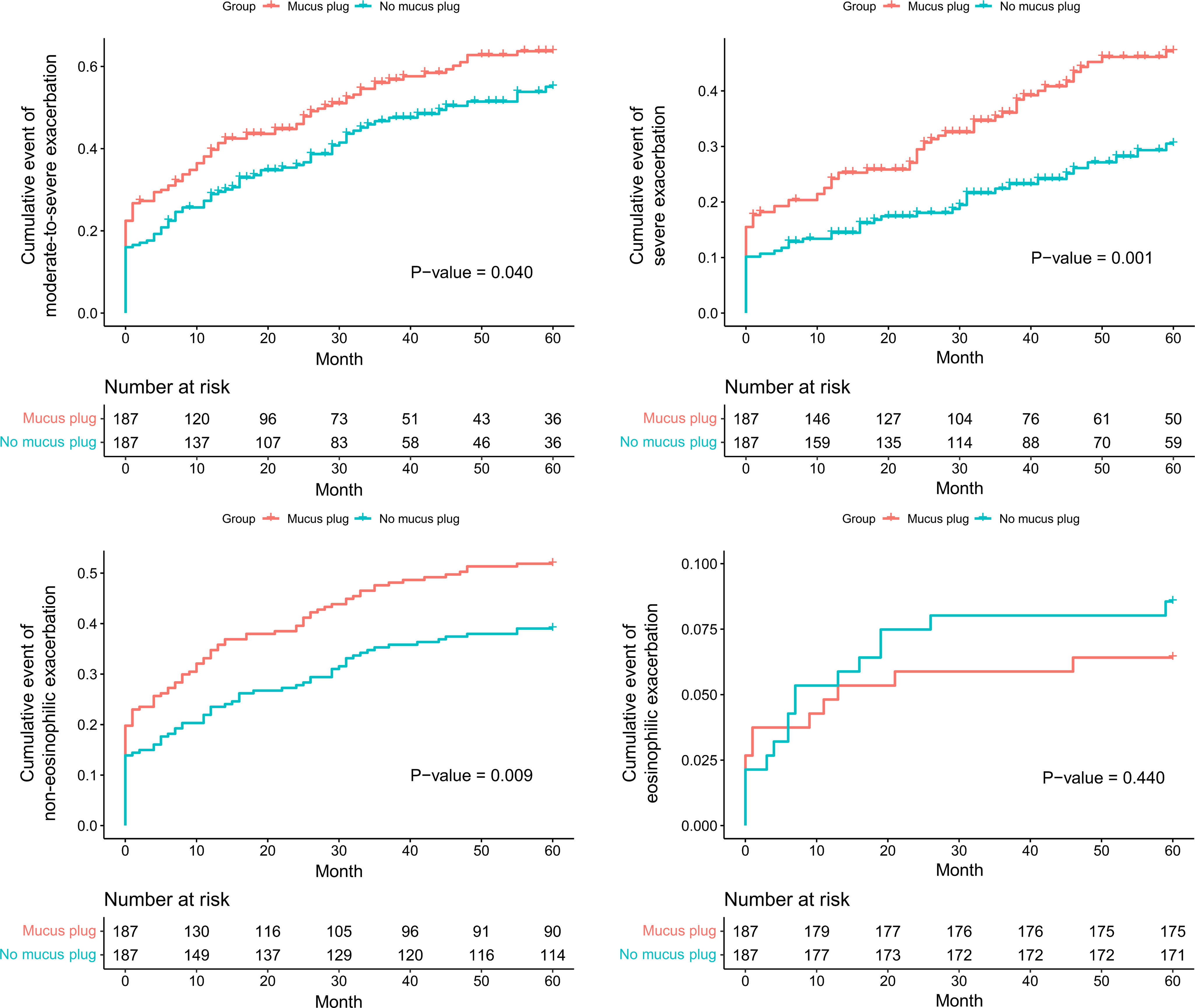
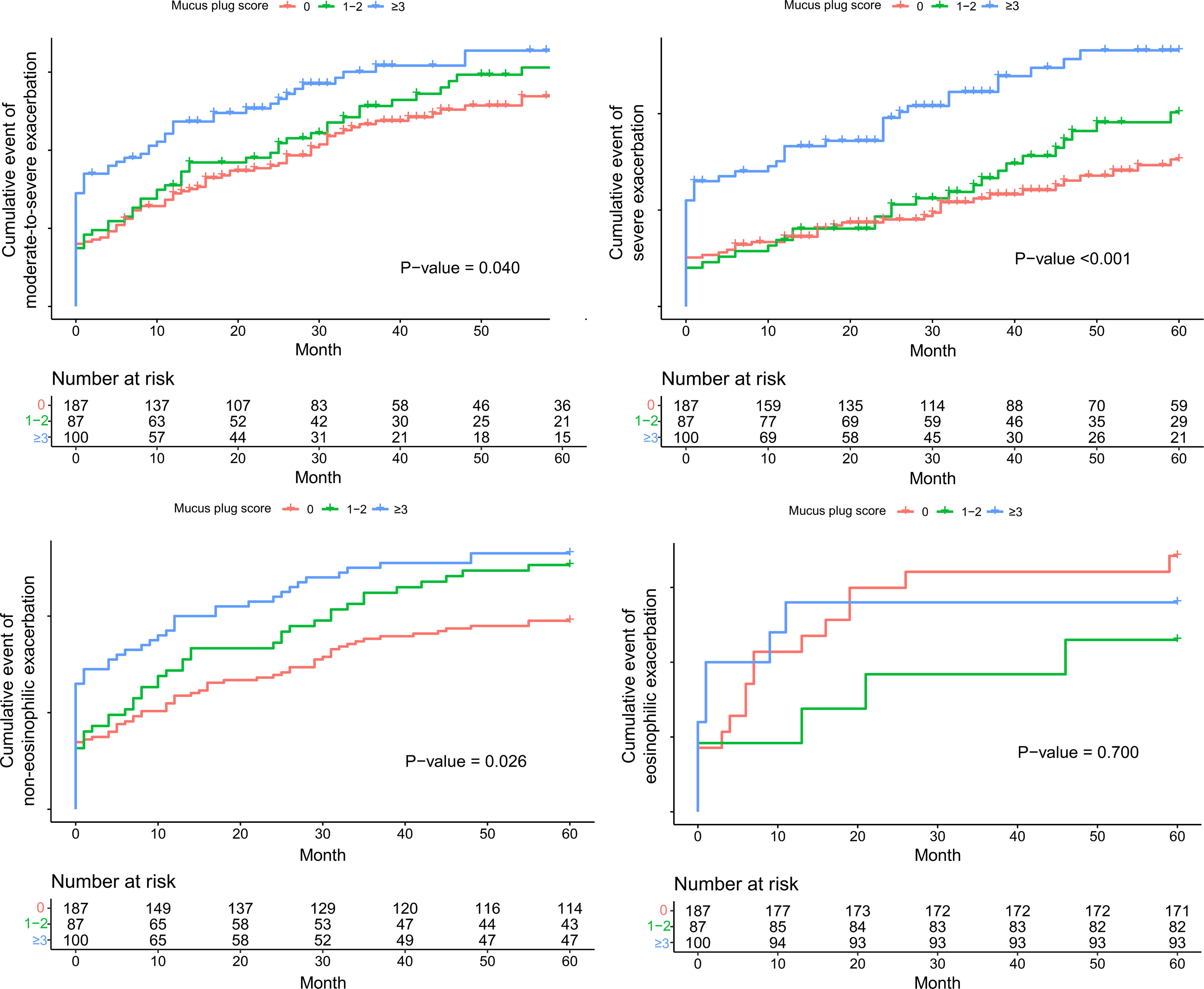
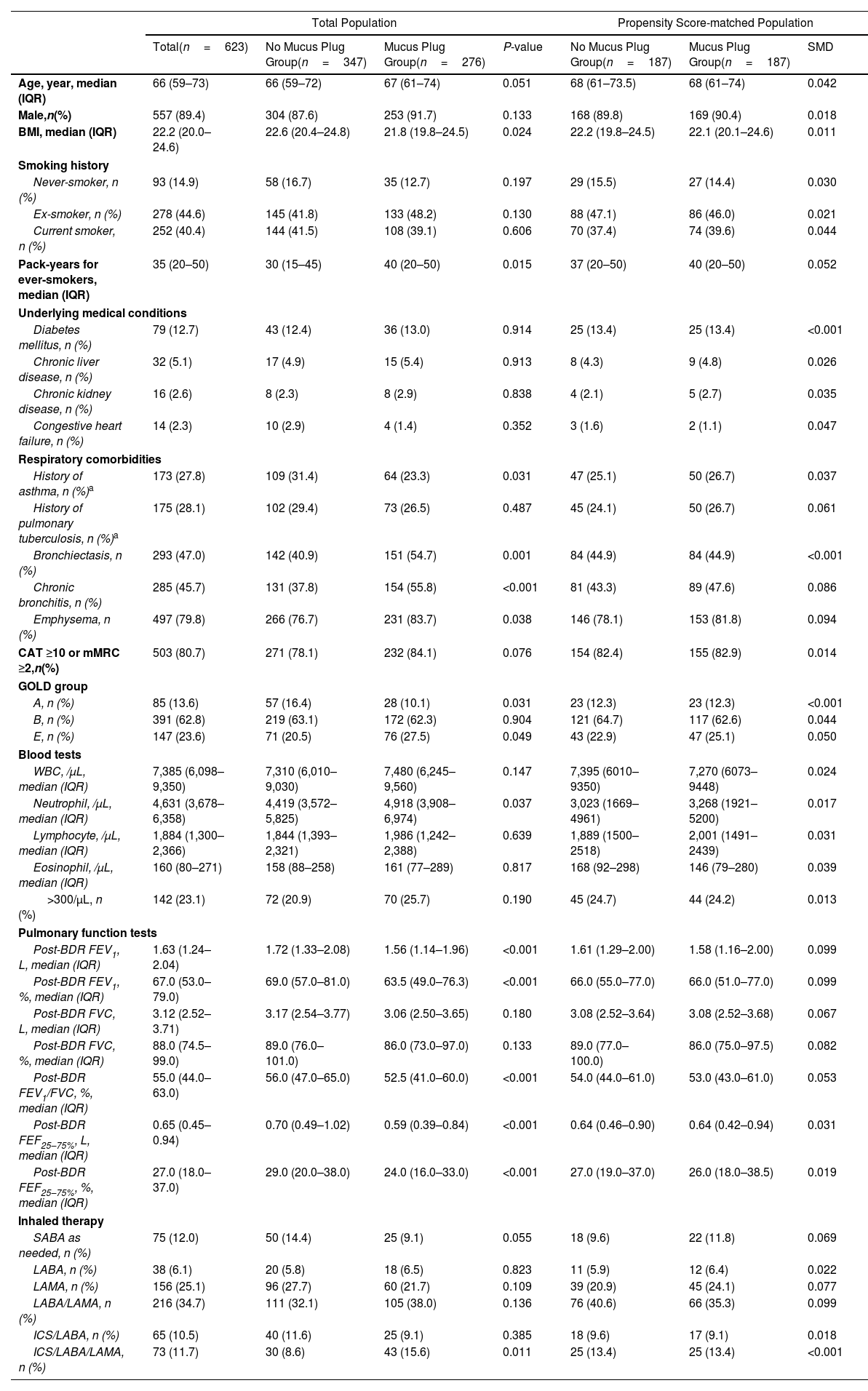
ResultsAmong 623 eligible patients, the mucus plug group was 44.3%. Through 1:1 propensity-score matching, each group was comprised of 187 individuals with balanced covariates. The mucus plug group showed higher rates of moderate-to-severe (0.51/year vs. 0.58/year, P=0.035), severe exacerbations (0.21/year vs. 0.24/year, P=0.032), and non-eosinophilic exacerbations (0.45/year vs. 0.52/year, P=0.008). Mucus plugs were associated with increased hazard of moderate-to-severe (adjusted HR=1.502 [95% CI 1.116–2.020]), severe (adjusted HR=2.106 [95% CI, 1.429–3.103]), and non-eosinophilic exacerbations (adjusted HR=1.551 [95% CI, 1.132–2.125]). Annual FEV1 decline was accelerated in the mucus plug group (β-coefficient=−62 [95% CI, −120 to −5], P=0.035). Sensitivity analysis showed higher risk of exacerbations and accelerated FEV1 decline in mucus plug score ≥3 compared to score 0.
ConclusionsMucus plugs are associated with increased risks of exacerbations, particularly non-eosinophilic, and accelerated FEV1 declines over 5 years. Our study identified the potential prognostic value of mucus plugs on future exacerbation risks and lung function decline trajectories.