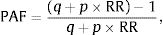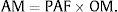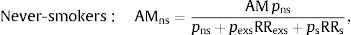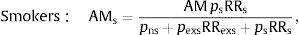Exposure to environmental tobacco smoke (ETS) is associated with increased mortality and morbidity. The objective of this study was to estimate the impact of ETS exposure in Spain on mortality in 2020 in the population aged 35 years and over.
MethodsA method of estimating attributable mortality (AM) based on the prevalence of ETS exposure was applied. Prevalence data were obtained from a representative study conducted in Spain and the relative risks were derived from a meta-analysis. AM point estimates are presented along with 95% confidence intervals (95% CI), calculated using a bootstrap naive procedure. AM, both overall and by smoking habit, was estimated for each combination of sex, age group, and cause of death (lung cancer and ischemic heart disease). A sensitivity analysis was performed.
ResultsA total of 747 (95% CI 676–825) deaths were attributable to ETS exposure, of which 279 (95% CI 256–306) were caused by lung cancer, and 468 (95% CI 417–523) by ischemic heart disease. Three-quarters (75.1%) of AM occurred in men and 60.9% in non-smokers. When chronic obstructive pulmonary disease and cerebrovascular disease are included, the burden of AM is estimated at 2242 deaths.
ConclusionsETS exposure is associated with 1.5% of all deaths from lung cancer and ischemic heart disease in the population aged 35 and over. These data underline the need for health authorities to focus on reducing exposure to ETS in all settings and environments.
More than 15 years ago, Spanish Act 28/2005, regulating the sale of cigarettes and smoking in public places, entered into force. This act was amended 5 years later on December 31, 2010 to extend the ban on smoking to the indoor areas of all leisure venues.1,2 Data from Spanish and European health surveys show that the overall prevalence of exposure to environmental tobacco smoke (ETS) among the Spanish population has remained stable since 2014, and the most recent results from 2020 confirm this trend.3 However, the results of other studies differ. Specifically, data from the 2020 Attitudes of Europeans towards Tobacco and Electronic Cigarettes Special Eurobarometer study show that in Spain the prevalence of indoor ETS exposure has increased compared with Eurobarometer 2017 data. In 2020, the prevalence of exposure in bars was estimated at 22%, 10 percentage points above the 2017 estimate and at 16% in restaurants, 13 points above the 2017 prevalence.4
To date, a causal relationship has been established between ETS exposure and mortality from lung cancer, ischemic heart disease, sudden infant death syndrome, chronic obstructive pulmonary disease (COPD) and cerebrovascular disease, although the data from the latter two are inconclusive.5 The latest study of the mortality burden attributable to ETS exposure in Spain estimated 1028 deaths in never-smokers in 2011, a similar figure to the previous study conducted in 2002.6,7 That was 20 years ago, and ETS-attributable mortality (AM) in Spain has not been recalculated since then.
The aim of this study was to estimate ETS-AM in a population aged 35 and over in Spain in 2020.
MethodA method of estimating ETS-AM based on the calculation of population attributable fractions (PAF) was used.8 The estimate was made according to STREAMS-p recommendations.9
Calculation ProcessFirstly, the PAF of ETS exposure was calculated from the following formula:
where p is the prevalence of ETS exposure, q=1−p, y RR is the risk of dying from lung cancer or ischemic heart disease observed in non-smokers exposed to ETS compared to non-exposed subjects.The AM was then estimated by multiplying the PAF by the observed mortality (OM):
The number of deaths attributable to ETS exposure was distributed according to smoking habit using the following formulas:
Former smokers: AMexs=AMpexs RRexspns + pexsRRexs + psRRs,where pns, pexs and pf are the prevalences of never-smokers, former smokers and smokers, respectively, and RRexs and RRs are the relative risks of mortality from lung cancer or ischemic heart disease in former smokers and smokers compared to never-smokers.AM, both overall and according to smoking habit, was estimated for each combination of sex, age group (35–54, 55–64, 65–74, 75 and older), and cause of death (lung cancer and ischemic heart disease). The results by age were distributed into 2 groups, 35–64 years and 65 years or older. AM point estimates were presented with 95% confidence intervals (95% CI), calculated by a naive bootstrap procedure using Efron's percentile method.
Sources of InformationMortality due to Lung Cancer and Ischemic Heart DiseaseDeaths for which the main cause listed was tracheal, lung and bronchial cancer (ICD-10 codes C33-34) and ischemic heart disease (ICD-10 codes I20-25) in the population aged 35 years and older were extracted by sex and age group from 2020 statistical microdata on mortality by cause of death from the National Institute of Statistics (INE).10
Prevalence of ETS Exposure and SmokingThe prevalence of ETS exposure and the prevalence of smokers, former smokers, and never-smokers were calculated in the population aged 35 years and older, by sex and age group, from microdata from the 2020 European Health Survey (EES2020). This survey, carried out by the Spanish Ministry of Health, Consumption and Social Welfare and the INE in the population aged 15 and over living in main family homes throughout Spain, collected health information on 4 main areas: sociodemographic, health status, use of health services, and health determinants. EES2020 fieldwork was conducted between July 2019 and July 2020 and 22,072 people aged 15 and over were interviewed.
The question used to estimate the prevalence of ETS exposure was: “How often are you exposed to tobacco smoke indoors? Consider only situations where other people are smoking”, and there were 4 possible answers: “every day”, “at least once a week (but not every day)”, “less than once a week,” and “never or almost never”. People who reported daily or weekly exposure were classified as exposed.
A smoker was defined as a person who smoked at the time of the survey, a former smoker was a person who had smoked but no longer smoked, and a never-smoker was one who had never smoked.
Relative RisksThe risk of dying from ischemic heart disease [1.27 (1.19–1.36)] and lung cancer [1.16 (1.03–1.3)] among never-smokers compared with unexposed never-smokers was extracted from the Surgeon General's Report.5 The relative risks of smokers and former smokers, by sex and age group, are derived from 5 US cohorts comprising nearly 1 million people over the age of 29 during the period 2000–2010.11
Sensitivity AnalysisTo assess the impact of risks on the estimated AM, the calculation was repeated using mortality risks previously applied to the estimates in Spain.12,13 Two alternative scenarios were also evaluated, one using the estimated chronic obstructive pulmonary disease (COPD)-AM and the other using estimated cerebrovascular disease (CVD)-AM, since the Surgeon General's Report suggests a likely causal relationship between ETS and both diseases. The OM for both causes of death, coded as the main cause, are derived from ICD-10 codes J40-44 and I60-69 recorded in 2020, and the risks used in the analysis are derived from the Fischer14 and Oono studies,15 respectively. Finally, the lung cancer- and ischemic heart disease-AM in never-smokers was estimated from the OM not attributable to smoking.
ResultsIn 2020, 11.5% of the Spanish population aged 35 years and older reported being exposed to ETS indoors, with the highest prevalence being recorded in men (12.5%) and in the age group aged 35–64 years, in both men and women. The highest prevalence, 33.9%, was among female smokers aged 35–64 years (Table 1).
Prevalence of Smoking and Exposure to Environmental Tobacco Smoke in the Population Aged 35 and Over, by Sex and Age Group. Spain 2020.
| Smoking (%) | Prevalence of Exposure to Environmental Tobacco Smoke (%) | ||||||
|---|---|---|---|---|---|---|---|
| Smokers | Former Smokers | Never-smokers, % | Overall | Smokers | Former Smokers | Never-smokers, % | |
| Men | 25.0 (23.9–26.2) | 34.1 (32.9–35.3) | 40.8 (39.6–42.1) | 12.5 (11.7–13.5) | 33.6 (31.2–36.2) | 6.7 (5.6–7.9) | 4.5 (3.6–5.6) |
| 35–64 years | 29.7 (28.3–31.2) | 27.7 (26.3–29.1) | 42.5 (41.0–44.1) | 14.7 (13.6–15.9) | 33.7 (21.3–36.5) | 8.7 (7.1–10.5) | 5.5 (4.4–6.9) |
| ≥65 years | 12.5 (11.1–14.1) | 51.2 (49.0–53.4) | 36.2 (34.1–38.4) | 6.6 (5.5–7.8) | 33.2 (27.3–39.6) | 3.8 (2.7–5.2) | 1.4 (0.8–2.6) |
| Women | 17.9 (16.9–18.9) | 19.7 (18.7–20.6) | 62.5 (61.3–63.7) | 10.5 (9.8–11.4) | 33.4 (30.6–36.3) | 8.4 (6.9–10.3) | 4.7 (4.0–5.4) |
| 35–64 years | 23.6 (22.3–25.0) | 23.4 (22.1–24.7) | 53.0 (51.4–54.6) | 13.2 (12.2–14.4) | 33.9 (30.1–37.0) | 9.1 (7.3–11.2) | 5.9 (4.9–7.1) |
| ≥65 years | 5.7 (4.9–6.7) | 11.8 (10.6–13.0) | 82.5 (81.0–83.9) | 4.8 (4.0–5.8) | 28.7 (21.9–36.6) | 5.8 (3.6–9.1) | 3.0 (2.3–4.0) |
In 2020 in Spain, 51,501 deaths in the population aged 35 years and over were caused by cancers of the trachea, lung and bronchi, and ischemic heart disease, 34,681 of which occurred in men. ETS exposure was attributable to 747 (95% CI 676–825) deaths in the population aged 35 and over, accounting for 1.5% of all deaths from lung cancer and ischemic heart disease (Fig. 1), of which 279 (95% CI 256–306) deaths were due to lung cancer and 468 (95% CI 417–523) to ischemic heart disease. Overall, 75.1% of AM occurred in men, with 561 deaths (95% CI 494–634) compared to 185 (95% CI 154–222) in women. Just under two-thirds (60.9%) of the deaths attributable to ETS exposure occurred in non-smokers: 260 (95% CI 228–297) in former smokers and 195 (95% CI 166–224) in never-smokers. The remaining 293 (95% CI 267–323) occurred in smokers (Table 2).
Population attributable fraction (PAF) (%) due to exposure to environmental tobacco smoke by lung cancer and ischemic heart disease, in total, by sex, by age group (35–64, 65 and above) and by sex and age group. The vertical line represents the total PAF for lung cancer and ischemic heart disease in the population aged 35 and over (1.5%).
Observed Mortality, Population Attributable Fraction and Environmental Tobacco Smoke-attributable Mortality Overall and According to Smoking Habit. Data are Presented by Cause of Death, Sex and Age Group.
| Observed Mortality | Population Attributable Fraction (%) | Mortality Attributable to ETS Exposure | ||||
|---|---|---|---|---|---|---|
| OverallN (95% CI) | SmokersN (95% CI) | Former SmokersN (95% CI) | Never-smokers, %N (95% CI) | |||
| Lung cancer | 280 (255.5–305.6) | 161 (147.7–177.6) | 96 (85.7–107.6) | 22 (19.9–24.2) | ||
| Men | 16,603 | 1.3 | 215 (190.9–240.3) | 123 (108.9–137.6) | 82 (71.4–92.7) | 11 (9.7–12.3) |
| 35–64 years | 4,364 | 2.1 | 93 (82.4–102.7) | 68 (59.8–76.0) | 20 (17.6–22.7) | 5 (4.3–5.4) |
| ≥65 years | 12,239 | 1.0 | 123 (102.7–144.2) | 55 (44.6–66.5) | 62 (51.9–72.1) | 6 (5.0–7.3) |
| Women | 5,297 | 1.2 | 64 (58.5–71.1) | 39 (34.7–43.2) | 14 (12.9–16.1) | 11 (9.5–12.9) |
| 35–64 years | 1,972 | 2.0 | 39 (35.4–44.0) | 28 (24.5–31.2) | 8 (6.9–9.1) | 4 (3.4–4.2) |
| ≥65 years | 3,325 | 0.8 | 25 (20.3–29.8) | 11 (8.6–13.8) | 7 (5.3–7.8) | 7 (5.8–9.1) |
| Ischemic heart disease | 468 (417.3–522.8) | 131 (119.2–145.7) | 163 (141.8–189.5) | 173 (146.2–200.8) | ||
| Men | 18,078 | 1.9 | 346 (303.3–394.1) | 112 (100.5–123.3) | 149 (127.8–174.2) | 85 (72.8–99.6) |
| 35–64 years | 3,855 | 3.6 | 140 (127.3–152.4) | 73 (65.1–80.5) | 39 (34.9–43.2) | 28 (25.3–30.7) |
| ≥65 years | 14,223 | 1.5 | 207 (166.0–252.3) | 40 (30.7–50.3) | 110 (88.7–133.8) | 57 (45.3–70.7) |
| Women | 11,523 | 1.1 | 121 (95.7–151.3) | 19 (16.3–22.6) | 15 (12.3–17.7) | 87 (65.6–112.7) |
| 35–64 years | 675 | 3.3 | 23 (20.4–25.0) | 11 (9.7–12.3) | 5 (4.3–5.4) | 7 (6.0–7.5) |
| ≥65 years | 10,848 | 0.9 | 99 (73.1–128.6) | 8 (5.8–11.1) | 10 (7.7–12.9) | 81 (58.8–106.1) |
| Total | 51,501 | 1.5 | 747 (676.2–825.3) | 293 (267.3–322.8) | 260 (228.1–296.9) | 195 (166.4–224.1) |
CI: confidence interval; ETS: environmental tobacco smoke.
When the risks published by Hackshaw et al. for lung cancer and by Law et al. for ischemic heart disease are used, the overall AM increases by 26.5%, with an estimated 945 (95% CI 856–1039) deaths attributable to ETS exposure, albeit with an increase in ischemic heart disease-AM and a decrease in lung cancer-AM. When the burden of mortality from COPD and CVD is included in the calculation, the estimated AM increases 3-fold to 2242 deaths. If AM is estimated from the OM in never-smokers, 141 deaths are attributable to ETS exposure, 53 fewer than in the original estimate (Table 3).
Sensitivity Analysis Taking into Account Alternative Scenarios Point Estimates of Attributable Mortality are Accompanied by 95% Confidence Intervals (95% CI) in Parentheses.
| Study Considerations | Alternative Scenarios | AM Alternate ScenarioN (95% CI) | Effect on Global AM |
|---|---|---|---|
| RR of ischemic heart disease derived from the Surgeon General's Report | Law et al.,12 RR 1.23 (95% CI 1.14–1.33) | 545 (495.5–597.7) | Increases AM by 35.5% |
| RR of lung cancer derived from the Surgeon General's Report | Hackshaw et al.,13 RR Men: 1.34 (95% CI 0.97–1.84), RR Women 1.24 (95% CI 1.13–1.36) | 400 (356.5–446.8) | Decreases AM by 9.1% |
| There is no evidence of an association with COPD | There is evidence of an association with COPD | 667 (585.5–780.2) | Increases AM by 89.3% |
| There is no evidence of an association with CVD | There is evidence of an association with COPD | 828 (714.7–951.2) | Increases AM by 110.8% |
| Include OM in smokers and former smokers for lung cancer and ischemic heart disease | Estimate OM in never-smokers | 141 | Decreases AM in never-smokers by 27.7% |
AM: attributable mortality; COPD: chronic obstructive pulmonary disease; CVD: cerebrovascular disease; RR: relative risk.
In Spain, in 2020, exposure to ETS caused 747 deaths in the population aged 35 and over; 80% of these deaths occurred in men and 60% in non-smokers. Almost two-thirds (62.6%) of all attributable deaths were due to ischemic heart disease, and the remaining were due to lung cancer.
Compared with the latest AM estimates for ETS in Spain in 2011, the mortality burden among never-smokers has decreased considerably,7 as seen from the analysis of smoking-AM.16
The impact of ETS exposure on mortality has been estimated 3 times in Spain. The first estimate dates from 1990 and addresses mortality in never-smokers married to smokers. In that study, lung cancer deaths were estimated at 89, 29 fewer than in the current study conducted in 2020.17 The previous estimate of AM in Spain, as noted above, was from 2011.7 The most notable difference between the 2011 estimate and the 2020 estimate is the decrease in the prevalence of ETS exposure. It should be noted that the 2011 prevalence figures were derived from a sample of 2500 adults aged 18 years and older who were asked in detail about exposure to ETS at home and at work. The prevalence data of the current study are derived, as already noted, from the EES2020 survey, which assesses the overall prevalence of exposure to ETS in enclosed spaces, so there are no estimates of exposure by type of setting. This is an important limitation of the EES2020, which extends to the latest National Health Surveys. Asking about global exposure does not allow us to characterize in detail the settings in which the population is exposed, and hampers any detailed analysis of AM. The questions included in the different studies assessing ETS exposure are known to vary,18 so it is difficult to compare estimates derived from studies using different sources of ETS exposure prevalence. However, to date, it has not been possible to establish a minimum set of questions or a common definition of exposure.
The most important difference between the 2011 and 2020 estimates for Spain is that smokers were included in the 2020 AM estimate. In previous studies, the authors had excluded smokers from ETS exposure impact estimations in order to obtain conservative estimates, but this approach does not take into account the possible synergistic effect of smoking plus ETS exposure in the causes of death studied. Indeed, the effect of ETS exposure on the risk of lung cancer among smokers is clear and significant.19,20 Furthermore, according to the results of the International Lung Cancer Consortium,20 the risk of a smoker not exposed to ETS developing lung cancer is 2.83 (2.48–3.22), while for an exposed smoker, it is 4.79 (4.32–5.32). The inclusion of smokers, or even former smokers, is nothing new.21–23 We must point out here that the inclusion of smokers in the estimation of ETS-AM is based on available evidence. The previous 2 AM estimates in Spain calculated the mortality burden in never-smokers,6,7 but to do so an approximate value had to be assigned to OM in never-smokers, since these data are not available in Spain. To this end, the AM in smokers and former smokers was extracted from the global OM, accepting the possible limitations of this estimate and obviating the synergistic effect of active smoking and passive exposure, since the applied risks were not adjusted for exposure to ETS. Despite the differences in the calculation processes, the sensitivity analysis clearly shows that the estimates hardly differ.
It is difficult to compare the results of this study with those recently conducted in other countries, since either the prevalence of exposure in other studies focuses on specific settings rather than overall exposure or different causes of death are reported.24–27 In any case, the ETS-AM burden in these studies is estimated to be close to 1% for the causes analyzed.
The estimates presented here may underestimate the ETS-AM burden for different reasons. Firstly, the prevalence of ETS exposure was derived from the EES2020 survey, which does not take account of ETS exposure in people who reported that they were rarely exposed. Questions in the EES2020 survey are less detailed and the scope is more limited, factors that may have led to some underreporting of true exposure, even though the validity of self-reported exposure measurements in surveys is acceptable. This is an appropriate moment to remind ourselves that there is no safe threshold for exposure to ETS. Secondly, in this study we have only included diseases listed in the Surgeon General's Report as causally associated with ETS exposure with the highest level of evidence (Level 1).5 To date, evidence on the causal relationship between ETS exposure and CVD or COPD is inconclusive, although the associations are biologically plausible and evidence is increasing.5 Including CVD and COPD in the AM estimate triples the mortality burden attributable to ETS exposure and would account for more than 2000 deaths per year. Moreover, the estimate refers to the adult population and does not include the impact on infant mortality associated with sudden infant death syndrome. Thirdly, we used the risks derived from the Surgeon General's Report, the most widely used source of risk in the estimation of smoking-AM. The use of these risks will result in a lower estimate AM. Moreover, when ETS-AM was estimated, the risk of exposed smokers and former smokers was assimilated into the risk of exposed never-smokers, even though the risk among smokers is approximately 4 times higher.20
This study has a number of limitations. The first is the estimation method itself, in which the induction period for causes of death associated with ETS exposure is not assessed, since prevalence of exposure and mortality are recorded at the same moment in time. The impact of this assumption could vary depending on the cause of death assessed, and could, in the case of ischemic heart disease for example, be limited due to the shorter induction period of this condition.28,29 With regard to relative risk, we should point out that the risks applied in the estimation of AM, except for lung cancer, derive mainly from studies conducted in non-European countries where the characteristics of the population, and therefore their exposure to ETS, could be different. However, they constitute the best available evidence and values are similar to those obtained in other studies.18 With regard to lung cancer, when risks were taken from the meta-analysis performed in the Surgeon General's Report in 2006, only data from studies conducted in Europe were used (see Table 7.4 of the report). Another limitation involves the year to which the estimates refer, i.e., 2020, the year of the SARS-CoV-2 pandemic. This situation may have affected both the prevalence estimate and OM. Finally, it should be noted that the definition of exposure refers exclusively to ETS and does not include exposure to residual or third-hand smoke, despite growing evidence of its noxious impact on health.30
The main advantage of this study is that it provides ETS exposure data across Spain. Another benefit is that for the first time in Spain, AM point estimates are presented with confidence intervals obtained using robust methods that minimize the high variability associated with the relatively small magnitude of the point estimates. The quality of death records in Spain adds to the accuracy of the estimates obtained. For example, in 2020, only 0.7% of deaths in individuals aged 35 and over were coded as ICD-10 R99, unknown cause. Furthermore, recommendations aimed at improving the quality of estimates in the attribution of mortality have been followed when calculating AM.9
A total of 747 deaths may not seem high, especially when compared with the mortality burden of 56,000 deaths attributable to smoking in Spain,31 but these figures account for 2 deaths every day in the population aged 35 years and over in Spain. We must emphasize that all these deaths are unnecessarily premature and preventable. Furthermore, these estimates refer to mortality, but we must not forget the important impact of ETS exposure on morbidity, especially asthma or otitis media in children.
In conclusion, exposure to ETS is an important risk factor that impacts mortality in Spain, due to both the magnitude of the risk and the persistent magnitude of exposure among the population. The greater part of the AM associated with ETS occurs in people who do not smoke. These data underline the need for health authorities at all administrative levels, and especially the Ministry of Health, to actively campaign for reducing exposure to ETS in the Spanish population in all settings.
Authors’ ContributionsMónica Pérez-Ríos: concept design, obtaining funding, writing original draft, editing; Diana Carolina López-Medina, Carla Guerra-Tort and María Isolina Santiago-Pérez: concept design, data analysis, critical review of the manuscript; Julia Rey-Brandariz: interpretation of study data and critical review of the manuscript; Leonor Varela-Lema: concept design, interpretation of study data and critical review of the manuscript; Cristina Candal, Agustin Montes, María José López, Regina Dalmau, Mariano Provencio, Ana Blanco and Esteve Fernández: interpretation of study data and critical review of the manuscript, Alberto Ruano-Ravina: concept design, interpretation of study data and critical review of the manuscript. All authors have read and approved the final manuscript.
FundingInstituto de Salud Carlos III (ISCIII), reference: PI22/00727, co-funded by the European Union.
Conflict of InterestsThe authors state that they have no conflict of interests.