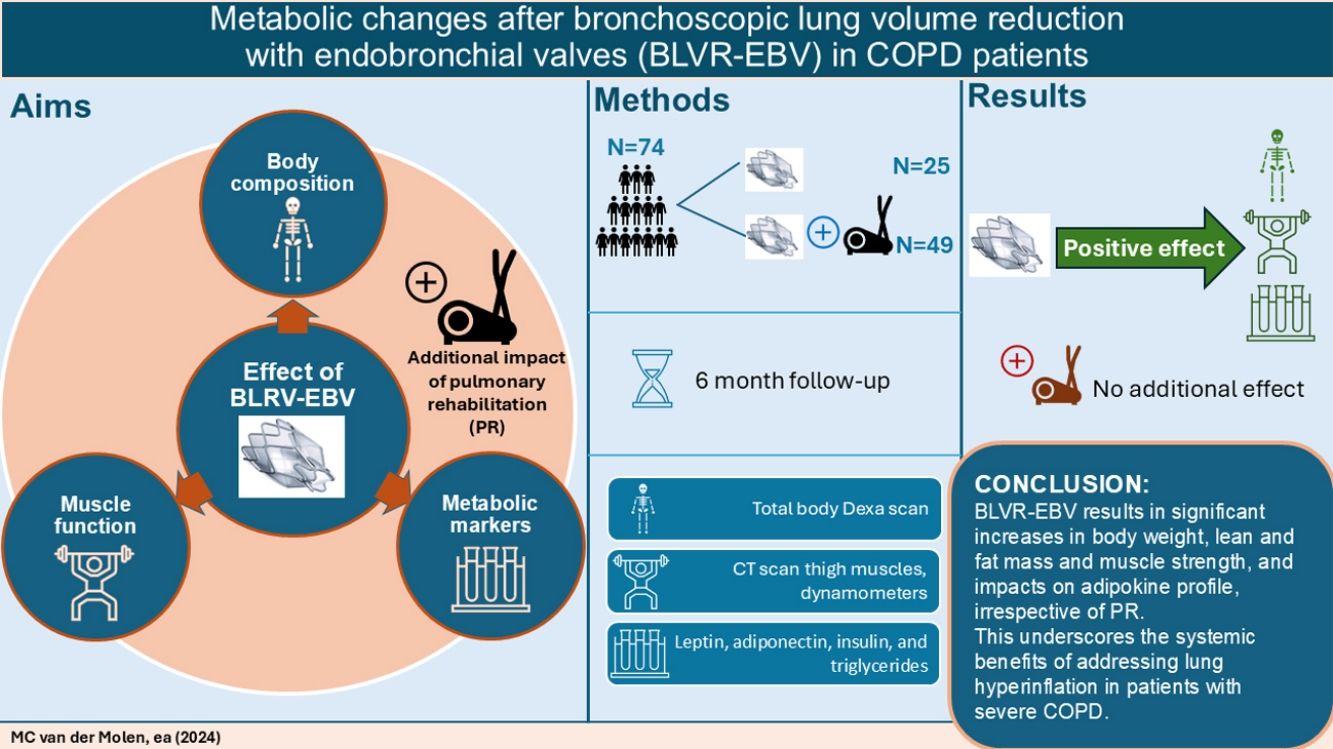
Little is known about the effect of bronchoscopic lung volume reduction using endobronchial valves (BLVR-EBV) on extrapulmonary manifestations like body composition, muscle function or metabolism. Pulmonary rehabilitation (PR) clearly addresses extrapulmonary manifestations of COPD, including physical inactivity and low muscle mass. However, the added impact of BLVR-EBV+PR remains unknown. Therefore, this study aimed to assess the effect of BLVR-EBV on body composition, muscle function and metabolic markers and whether PR has an additional impact on these outcomes.
MethodsSubjects with severe COPD eligible for both PR and BLVR-EBV were randomized into three groups: PR+BLVR-EBV, BLVR-EBV+PR, or only BLVR-EBV (n=97). Assessments included Dual Energy X-ray Absorptiometry, thigh muscle Computed Tomography, muscle strength measurements, accelerometry, and plasma (leptin, adiponectin, insulin, and triglycerides) at baseline and six months after the last intervention.
ResultsA total of 74 participants completed the study. At follow-up, there were significant increases in the groups combined and both groups separated in total weight, lean mass, fat mass, muscle strength, daily physical activity, and triglyceride levels while leptin/fat mass ratio levels were significantly reduced. No differences were found between groups who underwent BLVR-EVR alone or BLVR-EBV with PR.
ConclusionsBLVR-EBV results in significant increases in body weight, lean and fat mass, muscle strength and daily physical activity level, and impacts on adipokine profile, irrespective of PR. This underscores the systemic benefits of addressing lung hyperinflation in patients with severe COPD.
Chronic Obstructive Pulmonary Disease (COPD) is marked by persistent airflow limitation and ranks among the leading causes of death worldwide. In a subset of COPD patients characterized by severe emphysema and severe static lung hyperinflation, bronchoscopic lung volume reduction with endobronchial valves (BLVR-EBV) has emerged as a minimally invasive alternative to surgical procedures to reduce static lung hyperinflation, leading to improved lung function, exercise capacity, and quality of life.1
COPD is known to have multiple extrapulmonary manifestations, including abnormal body composition. characterized by low muscle mass and an excess of adipose tissue, particularly in the form of visceral fat.2,3 An abnormal body composition is frequently present among patients with COPD, and has been shown to negatively impact a patients’ morbidity and mortality.4,5 An excess of adipose tissue, and in particular visceral fat mass, is associated with enhanced systemic inflammation and reduced insulin sensitivity, thereby increasing the risk of cardiovascular diseases.6–8 Moreover, low body weight and unintentional weight loss are independent predictors of mortality in this population.9–12 Especially patients with emphysema and hyperinflation are prone to low body weight and low muscle mass.13
So far, limited is known about the effect of reducing hyperinflation on extrapulmonary manifestations like body composition, muscle function or metabolism. One study did find an increase in skeletal muscle mass after BLVR-EBV and after lung volume reduction surgery an increase was found in body mass index and fat-free mass index.14,15 Pulmonary rehabilitation (PR) has been shown to positively influence metabolism, body composition and muscle function, which is due to supervised resistance training, physical activity coaching in combination with nutritional supplements, as appropriate.2,16 The addition of pulmonary rehabilitation to BLVR-EBV could have a cumulative effect on these outcomes but this has never been investigated before to our knowledge. Therefore, this study aimed to assess the effect of BLVR-EBV on body composition, muscle function and metabolic markers and whether PR has an additional impact on these outcomes.
MethodsStudy designThis prospective study was performed as a predefined sub study of the multicenter Systemic effects of bronchoscopic Lung Volume reduction in severe Emphysema (SoLVE) trial (NCT03474471).17 The SoLVE trial involved 97 participants who were randomized into one of the following three groups: PR before BLVR-EBV (group 1), PR after BLVR-EBV (group 2), or BLVR-EBV without PR (group 3) at 2 sites (UMCG hospital Groningen or CIRO Horn). To investigate the additional effect of PR above BLVR-EVR we compared group 1 plus 2 versus group 3. Study visits were scheduled at baseline and 6 months after the last intervention (either PR or BLVR-EBV depending on group allocation). To be included in this sub study, patients must have completed both the baseline and follow-up visit. The study was approved by the local ethics committees at both sites (METc 2018/241), and written informed consent was obtained from all participants. Some of the baseline results were published before.17
Study participantsAll participants were ex-smokers, with severe emphysema and were considered eligible for BLVR-EBV and PR. Main inclusion criteria included forced expiratory volume in 1 second (FEV1)≤45% predicted, residual volume (RV)>175% predicted, emphysematous destruction of the target lobe>50% at −910Hounsfield Unit (HU), and a fissure integrity>95% (both on quantitative computed tomography analysis using LungQ software (Thirona, Nijmegen, The Netherlands)). Pulmonary function tests including spirometry, body plethysmography, and the 6-min walk test, were all performed in line with the current recommendations.18–20 An overview of all inclusion and exclusion criteria is provided in the online supplement (text E1).
Bronchoscopic lung volume reduction with endobronchial valvesIn BLVR-EBV, one-way endobronchial valves (Zephyr EBV, PulmonX, USA) were inserted into the segmental bronchi of a hyperinflated lung lobe. Endobronchial valves facilitate the exit of air from the treated lung lobe during exhalation, while preventing the entry of air during inhalation.21 As a result, the treated lung lobe deflates, leading to a reduction in hyperinflation.
All procedures were conducted in adherence to the most recent recommendations at either the University Medical Center Groningen, the Netherlands, or the Maastricht University Medical Center, the Netherlands.21
Pulmonary rehabilitationPR is a personalized and interdisciplinary program aimed at reducing symptoms and enhancing the physical and psychological well-being of patients with respiratory disorders.2 The program revolves around exercise training, but also entails nutritional support, education, and behavioral modifications. All PR programs in the study adhered to the ATS/ERS guidelines and had a mean duration of 8–10 weeks.2 Participants underwent a comprehensive PR program in one of the dedicated PR centers in the Netherlands: Beatrixoord (Haren), Dekkerswald (Nijmegen), Merem (Hilversum), Revant (Breda), or Ciro (Horn).
Dual energy X-ray absorptiometry (DEXA)Whole body composition was assessed using a total body scanner. Fat mass index and lean mass index were calculated as the total fat mass, or lean mass respectively, divided by squared height meters (kg/m2).
Computed tomography (CT) of the thigh musclesAnatomical images of the thigh muscles of both legs were obtained using a 64-slice CT scanner (SOMATOM Definition AS 64, Siemens, Germany) without intravenous contrast injection. Image acquisition was performed at a specific anatomic location, located at the midpoint between the lower edge of the pubic symphysis and the lower edge of the medial femoral condyle, as determined by femur scanography. The imaging procedure resulted in four 1.2mm-thick slices.
Skeletal muscle cross-sectional area, subcutaneous fat cross-sectional area, and the mean HU of the muscle cross-sectional were assessed, using Aquarius iNtuition (version 4.4.13.P6, TeraRecon), and the average of the four images was used for the analyses. Tissue ranging from −29 to +150HU was identified as muscle tissue, while tissue ranging from −190 and −30HU was classified as subcutaneous and intramuscular fat.22
Muscle strength, metabolic blood markers, and daily physical activityMaximal isometric muscle strength of the biceps, triceps and quadriceps was assessed using the MicroFET 2 handheld dynamometer (Hoggan Health Industries Inc., Salt Lake City, USA).23 Handgrip strength was measured with the JAMAR hydraulic hand dynamometer (Sammons Preston, Bolingbroke, USA).24 The average of the measures obtained from both sides was used for the analyses.
Blood samples were collected in a fasted state directly before BLVR-EBV and 6 months after the last intervention (either PR or BLVR-EBV), and stored at −80°C until processing. Plasma was utilized to measure concentrations of: leptin, adiponectin, insulin, and triglycerides. Since leptin and adiponectin are released by adipose tissue, also the ratios with correction for total fat mass were calculated. Daily physical activity was assessed using an accelerometer (DynaPort MoveMonitor, McRoberts BV, The Hague, the Netherlands) that was worn continuously for seven consecutive days.25
Statistical analysisThe statistical analysis of the data was carried out using IBM SPSS statistics (version 28, IBM, Armonk, USA), with statistical significance defined as a p-value less than 0.05. Paired t-tests (normal data distribution) or Wilcoxon signed rank tests (non-normal data distribution) were used to test the difference between baseline and follow-up, and independent t-tests (normal data distribution) or Mann–Whitney test (non-normal data distribution) to test for differences between the groups with and without PR. To examine differences in baseline data among the three study groups, we applied one-way ANOVA in case of normal data distribution and Kruskal–Wallis tests in case of non-normal data distribution.
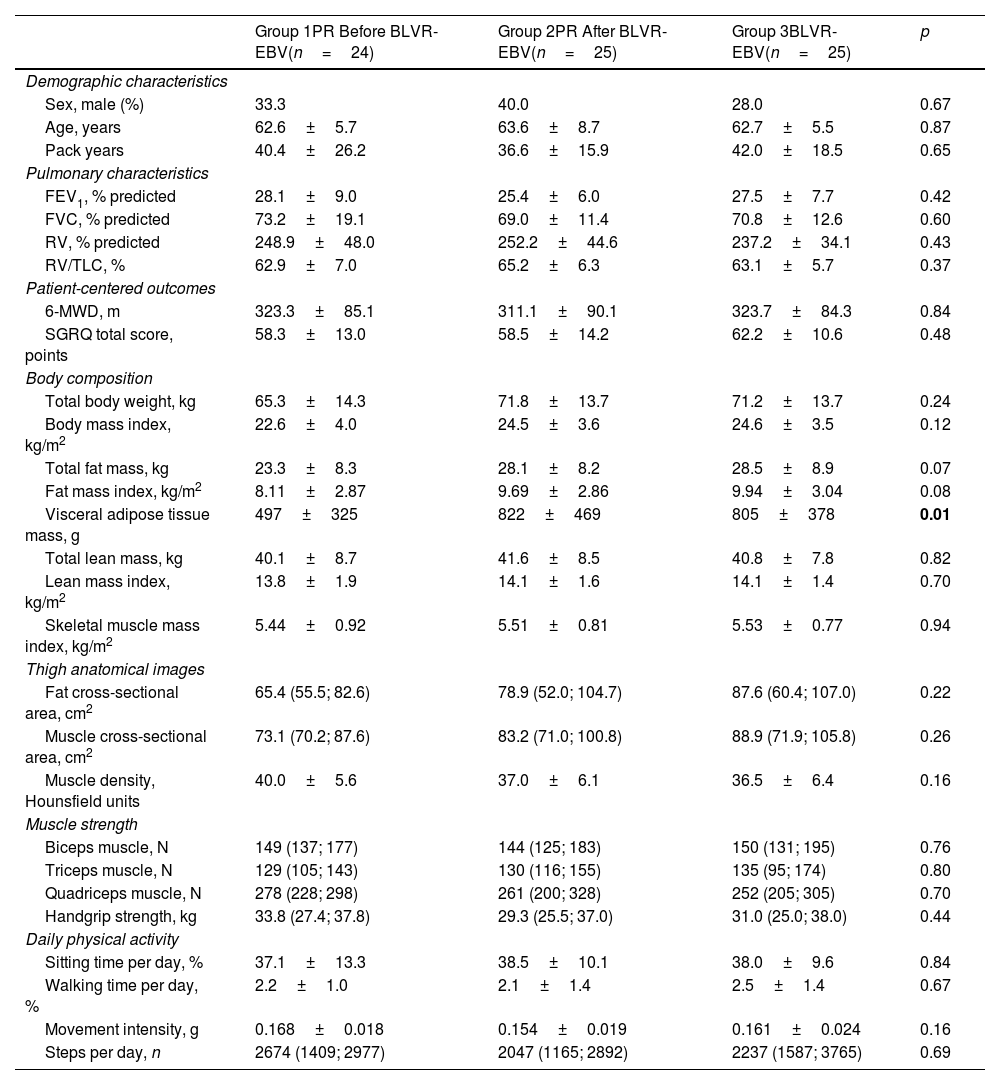
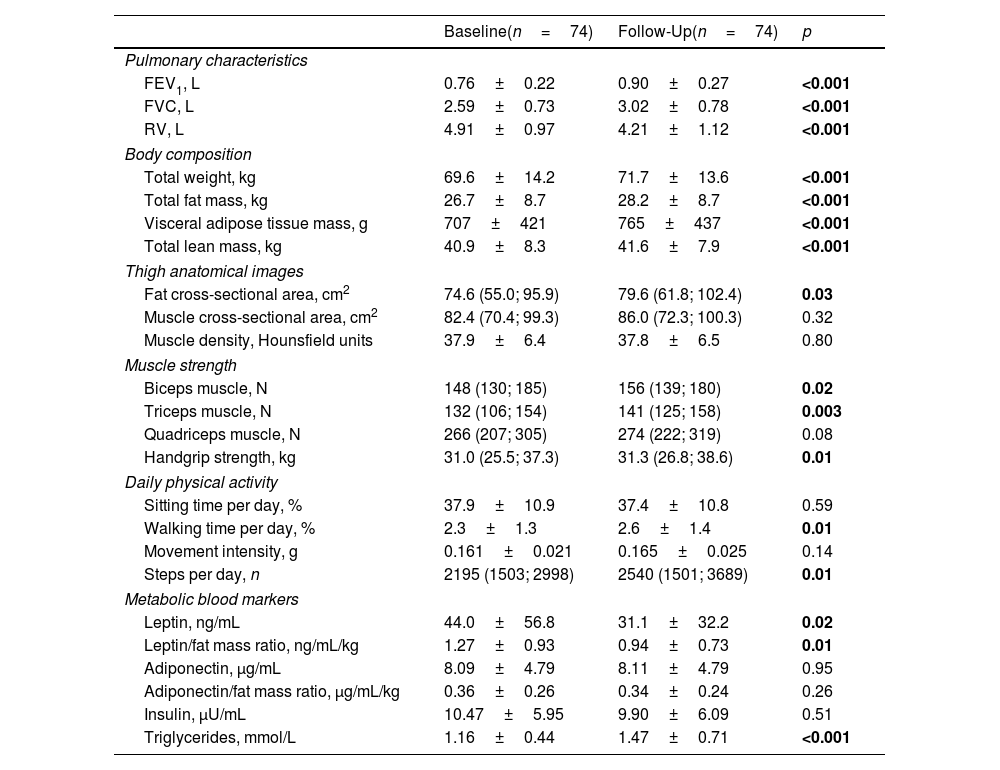
ResultsStudy populationOf the total 97 randomized participants, 74 participants completed the 6-month follow-up visit (see Table 1 for baseline characteristics). A flowchart of the study can be found in reference.17 This reference also demonstrated that there were no differences in baseline characteristics between groups, nor in the occurrence of adverse events.17 At the 6-month final visit, significant improvements in FEV1 and FVC were seen, along with a significant reduction in RV (Table 2).
Baseline Characteristics per Group.
| Group 1PR Before BLVR-EBV(n=24) | Group 2PR After BLVR-EBV(n=25) | Group 3BLVR-EBV(n=25) | p | |
|---|---|---|---|---|
| Demographic characteristics | ||||
| Sex, male (%) | 33.3 | 40.0 | 28.0 | 0.67 |
| Age, years | 62.6±5.7 | 63.6±8.7 | 62.7±5.5 | 0.87 |
| Pack years | 40.4±26.2 | 36.6±15.9 | 42.0±18.5 | 0.65 |
| Pulmonary characteristics | ||||
| FEV1, % predicted | 28.1±9.0 | 25.4±6.0 | 27.5±7.7 | 0.42 |
| FVC, % predicted | 73.2±19.1 | 69.0±11.4 | 70.8±12.6 | 0.60 |
| RV, % predicted | 248.9±48.0 | 252.2±44.6 | 237.2±34.1 | 0.43 |
| RV/TLC, % | 62.9±7.0 | 65.2±6.3 | 63.1±5.7 | 0.37 |
| Patient-centered outcomes | ||||
| 6-MWD, m | 323.3±85.1 | 311.1±90.1 | 323.7±84.3 | 0.84 |
| SGRQ total score, points | 58.3±13.0 | 58.5±14.2 | 62.2±10.6 | 0.48 |
| Body composition | ||||
| Total body weight, kg | 65.3±14.3 | 71.8±13.7 | 71.2±13.7 | 0.24 |
| Body mass index, kg/m2 | 22.6±4.0 | 24.5±3.6 | 24.6±3.5 | 0.12 |
| Total fat mass, kg | 23.3±8.3 | 28.1±8.2 | 28.5±8.9 | 0.07 |
| Fat mass index, kg/m2 | 8.11±2.87 | 9.69±2.86 | 9.94±3.04 | 0.08 |
| Visceral adipose tissue mass, g | 497±325 | 822±469 | 805±378 | 0.01 |
| Total lean mass, kg | 40.1±8.7 | 41.6±8.5 | 40.8±7.8 | 0.82 |
| Lean mass index, kg/m2 | 13.8±1.9 | 14.1±1.6 | 14.1±1.4 | 0.70 |
| Skeletal muscle mass index, kg/m2 | 5.44±0.92 | 5.51±0.81 | 5.53±0.77 | 0.94 |
| Thigh anatomical images | ||||
| Fat cross-sectional area, cm2 | 65.4 (55.5; 82.6) | 78.9 (52.0; 104.7) | 87.6 (60.4; 107.0) | 0.22 |
| Muscle cross-sectional area, cm2 | 73.1 (70.2; 87.6) | 83.2 (71.0; 100.8) | 88.9 (71.9; 105.8) | 0.26 |
| Muscle density, Hounsfield units | 40.0±5.6 | 37.0±6.1 | 36.5±6.4 | 0.16 |
| Muscle strength | ||||
| Biceps muscle, N | 149 (137; 177) | 144 (125; 183) | 150 (131; 195) | 0.76 |
| Triceps muscle, N | 129 (105; 143) | 130 (116; 155) | 135 (95; 174) | 0.80 |
| Quadriceps muscle, N | 278 (228; 298) | 261 (200; 328) | 252 (205; 305) | 0.70 |
| Handgrip strength, kg | 33.8 (27.4; 37.8) | 29.3 (25.5; 37.0) | 31.0 (25.0; 38.0) | 0.44 |
| Daily physical activity | ||||
| Sitting time per day, % | 37.1±13.3 | 38.5±10.1 | 38.0±9.6 | 0.84 |
| Walking time per day, % | 2.2±1.0 | 2.1±1.4 | 2.5±1.4 | 0.67 |
| Movement intensity, g | 0.168±0.018 | 0.154±0.019 | 0.161±0.024 | 0.16 |
| Steps per day, n | 2674 (1409; 2977) | 2047 (1165; 2892) | 2237 (1587; 3765) | 0.69 |
Data are displayed as mean±SD or percentage. BLVR-EBV=bronchoscopic lung volume reduction with endobronchial valves; PR=pulmonary rehabilitation; FEV1=forced expiratory volume in 1second; FVC=forced vital capacity, RV=residual volume; TLC=total lung capacity; %predicted: based on the GLI-2021 norms; 6-MWD=6-minute walking distance; SGRQ=St. George's Respiratory Questionnaire. One-way ANOVA and Kruskal–Wallis tests were performed to test for statistically significant differences between the groups.
Outcomes at Baseline and Six Months Follow-Up for All Participants.
| Baseline(n=74) | Follow-Up(n=74) | p | |
|---|---|---|---|
| Pulmonary characteristics | |||
| FEV1, L | 0.76±0.22 | 0.90±0.27 | <0.001 |
| FVC, L | 2.59±0.73 | 3.02±0.78 | <0.001 |
| RV, L | 4.91±0.97 | 4.21±1.12 | <0.001 |
| Body composition | |||
| Total weight, kg | 69.6±14.2 | 71.7±13.6 | <0.001 |
| Total fat mass, kg | 26.7±8.7 | 28.2±8.7 | <0.001 |
| Visceral adipose tissue mass, g | 707±421 | 765±437 | <0.001 |
| Total lean mass, kg | 40.9±8.3 | 41.6±7.9 | <0.001 |
| Thigh anatomical images | |||
| Fat cross-sectional area, cm2 | 74.6 (55.0; 95.9) | 79.6 (61.8; 102.4) | 0.03 |
| Muscle cross-sectional area, cm2 | 82.4 (70.4; 99.3) | 86.0 (72.3; 100.3) | 0.32 |
| Muscle density, Hounsfield units | 37.9±6.4 | 37.8±6.5 | 0.80 |
| Muscle strength | |||
| Biceps muscle, N | 148 (130; 185) | 156 (139; 180) | 0.02 |
| Triceps muscle, N | 132 (106; 154) | 141 (125; 158) | 0.003 |
| Quadriceps muscle, N | 266 (207; 305) | 274 (222; 319) | 0.08 |
| Handgrip strength, kg | 31.0 (25.5; 37.3) | 31.3 (26.8; 38.6) | 0.01 |
| Daily physical activity | |||
| Sitting time per day, % | 37.9±10.9 | 37.4±10.8 | 0.59 |
| Walking time per day, % | 2.3±1.3 | 2.6±1.4 | 0.01 |
| Movement intensity, g | 0.161±0.021 | 0.165±0.025 | 0.14 |
| Steps per day, n | 2195 (1503; 2998) | 2540 (1501; 3689) | 0.01 |
| Metabolic blood markers | |||
| Leptin, ng/mL | 44.0±56.8 | 31.1±32.2 | 0.02 |
| Leptin/fat mass ratio, ng/mL/kg | 1.27±0.93 | 0.94±0.73 | 0.01 |
| Adiponectin, μg/mL | 8.09±4.79 | 8.11±4.79 | 0.95 |
| Adiponectin/fat mass ratio, μg/mL/kg | 0.36±0.26 | 0.34±0.24 | 0.26 |
| Insulin, μU/mL | 10.47±5.95 | 9.90±6.09 | 0.51 |
| Triglycerides, mmol/L | 1.16±0.44 | 1.47±0.71 | <0.001 |
Data are displayed as mean±SD or median (IQR). FEV1=forced expiratory volume in 1 second; FVC=forced vital capacity, RV=residual volume; N=Newton; g=body acceleration. Paired t-tests or Wilcoxon signed rank tests were performed to test for statistically significant differences between the visits. Significant values are depicted in bold.
Among the participants who completed the study, 24 received PR+BLVR-EBV (group 1), 25 received BLVR-EBV+PR (group 2), and 25 underwent only BLVR-EBV (group 3). It is worth noting that three participants from group 2 chose not to follow PR, but did attend the follow-up visit. The mean follow-up duration from baseline to the follow-up visit was 11.2±2.1 months for group 1, 11.6±2.9 for group 2, and 8.9±2.6 months for group 3.
General changes in body compositionIn general, all patients grouped together demonstrated significant increases in all outcome measures assessed by DEXA, including total weight, as well as total fat and lean mass (Table 2). CT scans of the thigh muscles showed a significant increase in the fat cross-sectional area, but no significant changes in the muscle cross-sectional area and muscle density between baseline and follow-up (Table 2).
Changes in muscle strength and physical activityOverall, we observed significant increases in arm strength and hand grip strength, but not in strength of the quadriceps muscle (Table 2). Regarding daily physical activity, a significant increase in walking time and daily step count was found.
Metabolic blood markersAn overall significant reduction in leptin level, leptin level corrected for the total fat mass and increase in triglyceride level was found (Table 2). No differences were found in adiponectin level, adiponectin/fat mass ratio, and insulin level.
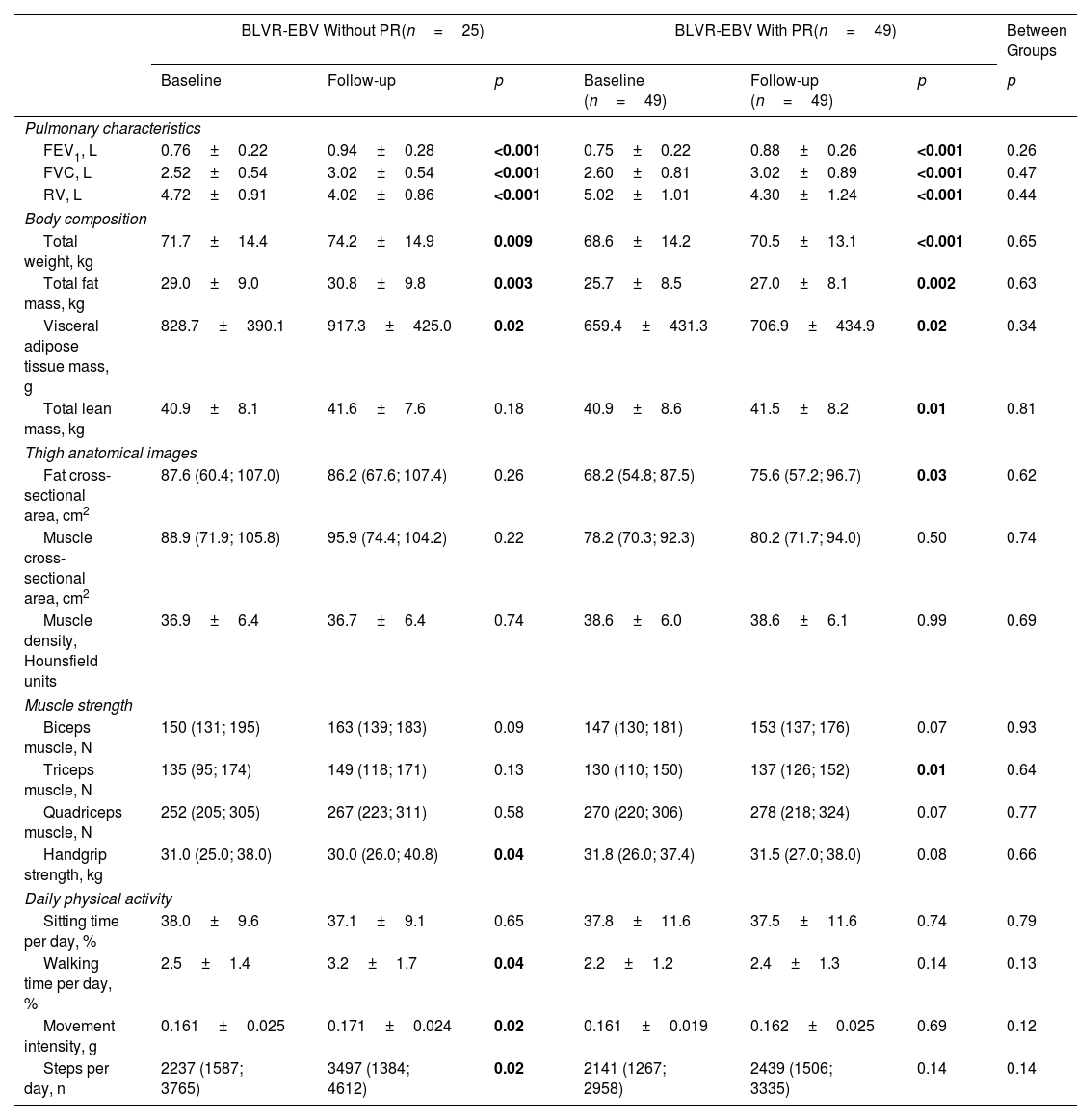
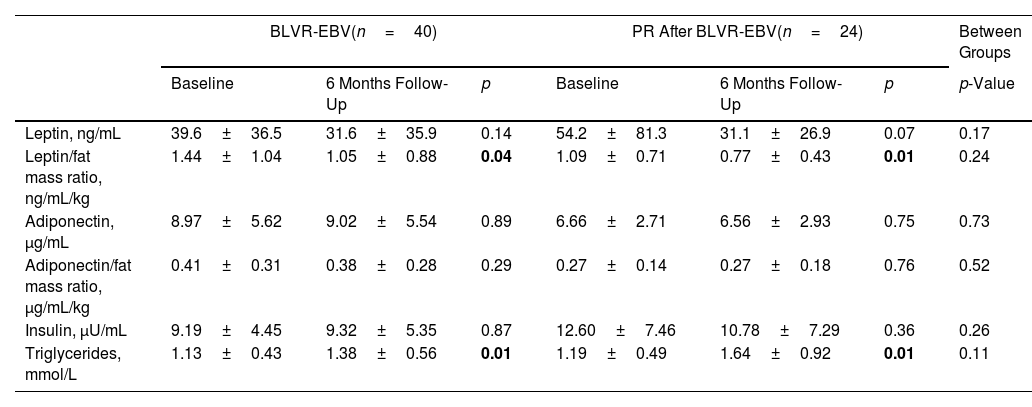
BLVR-EBV in combination with PR compared to BLVR-EBV aloneAcross all outcome measures related to body composition, muscle strength, physical activity and metabolic blood markers, we found no significant differences in changes in outcome between the combined group that underwent PR and those with BLVR-EBV alone (Tables 3 and 4). In addition, we observed no differences in changes in outcome measures comparing the three groups separately (Table E1 online supplement).
Changes in Outcomes Between Baseline and Follow-Up in Patients who Underwent Bronchoscopic Lung Volume Reduction With Endobronchial Valves Alone and in Combination With Pulmonary Rehabilitation.
| BLVR-EBV Without PR(n=25) | BLVR-EBV With PR(n=49) | Between Groups | |||||
|---|---|---|---|---|---|---|---|
| Baseline | Follow-up | p | Baseline (n=49) | Follow-up (n=49) | p | p | |
| Pulmonary characteristics | |||||||
| FEV1, L | 0.76±0.22 | 0.94±0.28 | <0.001 | 0.75±0.22 | 0.88±0.26 | <0.001 | 0.26 |
| FVC, L | 2.52±0.54 | 3.02±0.54 | <0.001 | 2.60±0.81 | 3.02±0.89 | <0.001 | 0.47 |
| RV, L | 4.72±0.91 | 4.02±0.86 | <0.001 | 5.02±1.01 | 4.30±1.24 | <0.001 | 0.44 |
| Body composition | |||||||
| Total weight, kg | 71.7±14.4 | 74.2±14.9 | 0.009 | 68.6±14.2 | 70.5±13.1 | <0.001 | 0.65 |
| Total fat mass, kg | 29.0±9.0 | 30.8±9.8 | 0.003 | 25.7±8.5 | 27.0±8.1 | 0.002 | 0.63 |
| Visceral adipose tissue mass, g | 828.7±390.1 | 917.3±425.0 | 0.02 | 659.4±431.3 | 706.9±434.9 | 0.02 | 0.34 |
| Total lean mass, kg | 40.9±8.1 | 41.6±7.6 | 0.18 | 40.9±8.6 | 41.5±8.2 | 0.01 | 0.81 |
| Thigh anatomical images | |||||||
| Fat cross-sectional area, cm2 | 87.6 (60.4; 107.0) | 86.2 (67.6; 107.4) | 0.26 | 68.2 (54.8; 87.5) | 75.6 (57.2; 96.7) | 0.03 | 0.62 |
| Muscle cross-sectional area, cm2 | 88.9 (71.9; 105.8) | 95.9 (74.4; 104.2) | 0.22 | 78.2 (70.3; 92.3) | 80.2 (71.7; 94.0) | 0.50 | 0.74 |
| Muscle density, Hounsfield units | 36.9±6.4 | 36.7±6.4 | 0.74 | 38.6±6.0 | 38.6±6.1 | 0.99 | 0.69 |
| Muscle strength | |||||||
| Biceps muscle, N | 150 (131; 195) | 163 (139; 183) | 0.09 | 147 (130; 181) | 153 (137; 176) | 0.07 | 0.93 |
| Triceps muscle, N | 135 (95; 174) | 149 (118; 171) | 0.13 | 130 (110; 150) | 137 (126; 152) | 0.01 | 0.64 |
| Quadriceps muscle, N | 252 (205; 305) | 267 (223; 311) | 0.58 | 270 (220; 306) | 278 (218; 324) | 0.07 | 0.77 |
| Handgrip strength, kg | 31.0 (25.0; 38.0) | 30.0 (26.0; 40.8) | 0.04 | 31.8 (26.0; 37.4) | 31.5 (27.0; 38.0) | 0.08 | 0.66 |
| Daily physical activity | |||||||
| Sitting time per day, % | 38.0±9.6 | 37.1±9.1 | 0.65 | 37.8±11.6 | 37.5±11.6 | 0.74 | 0.79 |
| Walking time per day, % | 2.5±1.4 | 3.2±1.7 | 0.04 | 2.2±1.2 | 2.4±1.3 | 0.14 | 0.13 |
| Movement intensity, g | 0.161±0.025 | 0.171±0.024 | 0.02 | 0.161±0.019 | 0.162±0.025 | 0.69 | 0.12 |
| Steps per day, n | 2237 (1587; 3765) | 3497 (1384; 4612) | 0.02 | 2141 (1267; 2958) | 2439 (1506; 3335) | 0.14 | 0.14 |
Data are displayed as mean±SD, median (IQR), or percentage. BLVR-EBV=bronchoscopic lung volume reduction with endobronchial valves; PR=pulmonary rehabilitation; FEV1=forced expiratory volume in 1second; FVC=forced vital capacity, RV=residual volume; N=Newton; g=body acceleration. Paired t-tests or Wilcoxon signed rank tests were performed to test for statistically significant differences between the visits within groups and independent t-tests were performed to test for statistically significant difference in change between groups.
Changes in Metabolic Blood Markers.
| BLVR-EBV(n=40) | PR After BLVR-EBV(n=24) | Between Groups | |||||
|---|---|---|---|---|---|---|---|
| Baseline | 6 Months Follow-Up | p | Baseline | 6 Months Follow-Up | p | p-Value | |
| Leptin, ng/mL | 39.6±36.5 | 31.6±35.9 | 0.14 | 54.2±81.3 | 31.1±26.9 | 0.07 | 0.17 |
| Leptin/fat mass ratio, ng/mL/kg | 1.44±1.04 | 1.05±0.88 | 0.04 | 1.09±0.71 | 0.77±0.43 | 0.01 | 0.24 |
| Adiponectin, μg/mL | 8.97±5.62 | 9.02±5.54 | 0.89 | 6.66±2.71 | 6.56±2.93 | 0.75 | 0.73 |
| Adiponectin/fat mass ratio, μg/mL/kg | 0.41±0.31 | 0.38±0.28 | 0.29 | 0.27±0.14 | 0.27±0.18 | 0.76 | 0.52 |
| Insulin, μU/mL | 9.19±4.45 | 9.32±5.35 | 0.87 | 12.60±7.46 | 10.78±7.29 | 0.36 | 0.26 |
| Triglycerides, mmol/L | 1.13±0.43 | 1.38±0.56 | 0.01 | 1.19±0.49 | 1.64±0.92 | 0.01 | 0.11 |
Data are displayed as mean±SD. BLVR-EBV=bronchoscopic lung volume reduction with endobronchial valves; PR=pulmonary rehabilitation. Paired t-tests were performed to test for statistically significant differences between the visits within groups and independent t-test to test for statistically significant difference between groups.
To our knowledge, this is the first study to examine the impact of BLVR-EBV as a standalone intervention compared to a combination with PR on alterations in body composition, muscle strength, physical activity, and blood markers. Our findings demonstrate that reducing hyperinflation by BLVR-EBV leads to substantial increases in body weight, lean mass, fat mass (including visceral fat mass), as well as muscle strength and physical activity, and impacts on adipokine profile, regardless of PR and its timing relative to BLVR-EBV.
We observed an overall increase in body weight and lean mass. In the context of COPD, unintended weight loss and a low body weight have been associated with increased mortality.9,10 Conversely, weight gain in patients with a BMI below 25 has been shown to improve survival outcomes, and patients with moderate levels of obesity exhibit lower mortality rates compared to those with a BMI below 25 (the so called ‘obesity paradox’).9,26,27 However, it has been suggested that lean body mass serves as a better predictor for survival compared to total body weight.11,12,28 Earlier findings have demonstrated that the presence of cachexia reduced the median survival by nearly half, irrespective of the extent of airflow obstruction.28 In addition to an increase in lean mass, we also observed a rise in fat mass, including visceral fat. It is well known that obesity – and in particular, excessive visceral fat – is strongly associated with insulin resistance, hypertension, and dyslipidaemia. And also in COPD, these metabolic disorders are known to contribute to high rates of mortality and morbidity, potentially through the involvement of fat tissue in promoting low-grade systemic inflammation.29 The question that emerges from our findings is whether the increases in fat mass, including visceral fat, translate into increased health risks in this patient group.
Several underlying factors have been described that can result in increased energy requirements and that may result in weight loss and a deteriorated nutritional state in patients with COPD, such as higher energy expenditure both at rest and during activity, disuse muscle atrophy, low-grade systemic inflammation, arterial hypoxemia, and hormonal insufficiency.30 In our study population, it is conceivable that a portion of the weight gain can be explained by lower resting energy expenditures as a result of reduced work of breathing due to improved respiratory mechanics following BLVR-EBV. But also loss of appetite due to systemic inflammation has been described in COPD, along with a reduced dietary intake to reduce symptoms of dyspnea.31 In line, previously, it was shown that after EBV treatment meal-related dyspnea decreased.32 Furthermore, it has been shown that lung volume reduction surgery leads to increased BMI, lean mass, and fat mass, and decreased resting energy expenditure and oxygen consumption.14,33 In lung volume reduction surgery, these decreases in resting energy expenditure and oxygen volume consumption were associated with a decrease in RV and increases in FEV1 and BMI.33 Although it is reasonable to expect similar findings in BLVR-EBV as observed in lung volume reduction surgery, this was not evident in a previous study involving a small group of patients who underwent BLVR-EBV.32 Therefore, additional research is needed to evaluate the impact of BLVR-EBV on resting energy expenditure in this population.
It is tempting to speculate that the increase in lean mass may be attributed to an increase in muscle function as a result of enhanced physical activities prompted by the improved lung function following BLVR-EBV. However, despite increases in muscle mass and strength, no changes in muscle density were found. Muscle density serves as an indicator of muscle quality, with lower attenuation scores indicating higher levels of intramuscular fat deposition.34 Although against conventional expectations, these findings are consistent with what was observed on lumbar level CT scans in patients who underwent BLVR-EBV.15 Notwithstanding the relatively large improvements in exercise capacity and muscle strength, it could be that there is still an insufficient training stimulus to effectively enhance muscle quality, particularly when considering the persistent very inactive lifestyle in this patient group. Another possible contributing factor is that in patients with COPD, a catabolic state due to an enhanced protein breakdown has been found, possibly attributed to low-grade systemic inflammation.30 But it is also possible that there may be other aspects of muscle quality improvement that a CT scan does not capture, such as enhanced blood circulation or a decrease in the proportion of type II muscle fibers.
In our study, we found a decline in leptin/fat mass ratio at follow-up. Leptin is a pro-inflammatory cytokine that regulates body weight by enhancing energy expenditure through activity-independent thermogenesis and reducing food intake by inducing satiety.35 Leptin/fat mass ratios have been inversely associated with dietary intake and body weight changes after nutritional interventions in patients with emphysema.36 Although our findings of lower leptin/fat mass ratios and increases in body weight seem compatible with each other, the mechanism behind the decrease in leptin/fat mass ratio after the interventions remains unclear. Given that leptin levels are known to increase in obesity and systemic inflammation, it might be hypothesized that inflammation becomes less pronounced following BLVR-EBV.35 However, data on this are lacking, and further research would be needed to investigate this. Adiponectin exhibits contrasting effects compared to leptin. Adiponectin is an anti-inflammatory cytokine that plays a protective role in the onset of insulin resistance, and adiponectin levels decrease in obesity, potentially due to systemic inflammation.37 Despite the increases in fat and visceral fat mass, we found no changes in adiponectin levels. This might align with an earlier hypothesis suggesting varying roles for adiponectin and leptin in COPD, potentially dependent on the phenotype, with a more pronounced impact of leptin in patients with emphysema.38 We found no changes in serum insulin level. Besides an inactive lifestyle, insulin resistance has also been described as a response to hypoxemia, systemic inflammation, and the use of glucocorticoids in the context of COPD.39
In our study, we observed an overall increase in triglyceride levels, approaching normal values at follow-up. In severe COPD, lower triglyceride levels have been described compared to those without COPD or with less advanced stages of the disease.40 Possible explanations for these changes in triglyceride levels include an increased dietary intake or metabolic shift from lipid catabolism to the utilization of glucose for energy production after the treatments.41
In our study we did not find that the addition of PR resulted in further improvements in body composition. While this does not imply that PR lacks an effect on body composition in general, it did not provide an additional benefit beyond the BLVR-EBV treatment. The effect of PR on body composition is variable and not extensively studied in the literature. One recent study investigated the effect of a PR program with additional dietary counseling and found a statistically significant decrease in fat mass between week 10 and 3 months after starting rehabilitation, but no other changes in body composition outcomes.42 Another randomized controlled trial compared the effects of PR with and without the addition of anabolic steroids on body composition, where the ‘regular’ PR group did not show a change in fat-free mass but did show a statistical significant increase in fat mass. Adding anabolic steroids resulted in a significant increase in fat-free mass, though no significant change in fat-mass was observed.43 A third study demonstrated that exercise alone did not lead to significant changes in body composition, but combining with either nutritional support or anabolic steroids led to improvements in body composition.44
In contrast to our study, one study found that PR prior to lung volume reduction surgery (LVRS) was effective.45 However, in that cohort, all patients underwent PR before LVRS, and there was no comparison group that did not follow a PR program Additionally, the impact of PR on the effectiveness of LVRS was not investigated, nor were changes in body composition or muscle function assessed. A key distinction with bronchoscopic treatment is that it is less invasive than surgery, making the requirement for patients to be ‘fit for surgery’ less critical for those undergoing a bronchoscopic procedure.
Our study has limitations. A limitation of our study is that the groups that underwent both BLVR-EBV and PR had a longer follow-up time (mean: 11.2 or 11.6 months) in comparison to the group that only underwent BLVR-EBV (mean: 8.9 months). The effects of PR are known to wane over time, which might have negatively impacted the results of the group that underwent PR before BLVR-EBV.46 Another limitation is that baseline blood samples for the group that underwent PR before BLVR-EBV were taken before BLVR-EBV for patient convenience, making it impossible to compare the three groups as intended in the study design. Finally, it would have been interesting to include a fourth group of patients who underwent pulmonary rehabilitation alone.
In conclusion, we found that BLVR-EBV leads to substantial increases in body weight, lean mass, fat mass (including visceral fat mass), as well as muscle strength, but not muscle density. Furthermore, our results showed that BLVR-EBV impacts the adipokine profile. The addition of a PR program before or after BLVR-EBV did not lead to additional benefits. This underscores the systemic benefits of addressing lung hyperinflation in patients with severe COPD.
Artificial Intelligence InvolvementNone of the material was produced with help of any artificial intelligence software.
Funding StatementThis study was funded by Lung Foundation Netherlands (grant number 5.1.17.171.0). The funder was not involved in the study design, conduct of the trial, data collection or analysis, interpretation, manuscript preparation, or decision to submit the manuscript for publication.
Conflict of InterestMCvdM, JEH, AV, HRG. TPW, ARV and RP have nothing to disclose. LEGWV reports to have received a research grant for this work from the Dutch Lung Foundation, grants or contracts from The Family Kamprad Foundation (20190024), the Swedish government and country council ALF grant (ALFGBG-824371), The Swedish Heart and Lung Foundation (20200150), Svensk Lungmedicinsk Förening (all paid to institution) and payment or honoraria for lectures, presentation, speakers, bureaus, manuscript writing or educational events from GSK, Astrazeneca, Boehringer, Novartis, Chiesi, Resmed, Pulmonx, Grifols (paid personally). DJS reports to have received grants or contracts from Pulmonx corp, USA, Nuvaira, USA, PulmAir, USA, Apreo, USA and FreeFlowmedical (clinical trial expenses), consulting fees from Nuvaira USA, MoreAir USA, Apreo USA and PulmonX USA (all paid to institution), payment or honoraria for lectures, presentation, speakers, bureaus, manuscript writing or educational events from PulmonX USA, Nuvaira USA (paid to institution), and support for attending meetings and/or travel from PulmonX USA. MS reports to have received grants or contracts from Netherlands Lung Foundation, Stichting Astma Bestrijding, Astra Zeneca, Boehringer Ingelheim, Chiesi, TEVA and Sanofi and consulting fees from AstraZeneca, Boehringer Ingelheim, and GSK.
Data StatementThe data that support the findings of this study are available from the corresponding author upon reasonable request.
We would like to thank our colleagues of the SoLVE consortium at the UMCG, MUMC+, and all five rehabilitation centers for their assistance in scheduling and conducting the treatments of our study participants.
University Medical Center Groningen, Groningen, the Netherlands – Marieke C. van der Molen, Jorine E. Hartman, Dirk-Jan Slebos, Marlies van Dijk, T. David Koster, Karin Klooster, Sonja W.S. Augustijn; Center of Rehabilitation Beatrixoord, Haren, the Netherlands – Hester van der Vaart; Merem Medical Rehabilitation, Hilversum, the Netherlands – Eline bij de Vaate; Radboud Institute of Health Sciences, Nijmegen, the Netherlands – Bram van den Borst; Pulmonary Rehabilitation Center Revant – Dirk van Ranst; Maastricht University Medical Center – Rein Posthuma, Kim H.M. Walraven; Ciro, Horn, the Netherlands – Anouk W. Vaes, Martijn A. Spruit; Sahlgrenska University Hospital, Gothenburg, Sweden – Lowie E.G.W. Vanfleteren.