Lung cancer (LC) and Chronic Obstructive Pulmonary Disease (COPD) are two highly prevalent smoking-related conditions which are associated with a high burden of morbidity and mortality worldwide.1 COPD patients are at increased risk of developing LC, which is major source of premature death.2
Evidence from two large randomized controlled trials confirms the survival gains from LC screening. The National Lung Screening Trial (NLST)3 showed a reduction in LC deaths of 16–20% with annual computed tomography (CT) screening vs. Chest X-ray screening, and The Dutch-Belgian study (NELSON)4 reported a reduction in LC deaths of about 30–40% (26% in men and 40–60% in women) in their screening group. Despite this strong evidence, implementation and uptake of LC screening programs in USA and Europe has been slow.
Unfortunately, evidence on the potential impact of LC screening in COPD patients is limited and mainly based on secondary analyses of prior studies. Our group was one of the first that explore this issue in a sample of COPD patients from the BODE cohort and showed a significant reduction in LC mortality incidence density in the screening vs. control group (0.08 vs. 2.48 per 100 person-years, p<0.001), with a number needed to screen to avoid one LC death of only 34.5 Young et al.6 also explore the impact of COPD (based on evidence of airway obstruction) in a subset of the NLST – cohort that underwent a spirometry (American College of Radiology Imaging Network: ACRIN cohort). They found that COPD status was associated with a doubling in LC incidence, no apparent over diagnosis, and a more favorable stage shift with screening. In a subsequent analysis,7 the authors reported on the impact of screening in the NLST-ACRIN sub study and found that the reduction of LC mortality was greater (28%) in those without than in with (15%) airway obstruction (AO). When the impact of screening was evaluated according to degree of AO, LC mortality was reduced 40% in those with spirometric GOLD stages 1 and 2 vs. 32% in those with undiagnosed COPD (individuals with AO in the screening spirometry but without a previous diagnosis of COPD). Lastly,8 we also investigated the potential impact of LC screening on patients with AO in the NLST-ACRIN cohort. We found that during the 1st year, there were no differences in mortality between the screening and control arms (p=0.65). However, thereafter, LDCT significantly decreased LC mortality (hazard ratio: 0.63, 95% confidence interval: 0.44–0.91). The number needed to screen to avoid one LC death in these subjects was 108 while in those without OLD was 218. We concluded that LC screening with LDCT in smokers with AO showed a trend to reduce lung cancer mortality but a study with a larger number of patients would be needed to confirm these findings.
Results from multiple studies indicate that the risk of LC increases with worsening AO severity.10 CT-detected emphysema confers additive risk of LC.11 Our group developed and validated the COPD Lung Cancer Screening Score (LUCSS),12 a composite index based on age>60 years, BMI<25kg/m2, pack years history>60, and emphysema on chest CT, that can be used to predict the risk of LC in patients with COPD. Patients in the high-risk category (≥7 points) have a higher risk of developing LC (hazard ratio [HR]: 3.5; 95% confidence interval [CI]: 1.7–7.1). To improve applicability in routine practice, we substituted emphysema for a diffusing lung capacity for carbon monoxide (DLCO)<60% of predicted, creating the validated the COPD-LUCSS-DLCO.13 A COPD-LUCSS-DLCO score between 3.5 and 8 points is associated with an increased risk of death from LC (HR: 2.4, 95% CI: 2.0–2.7).
A challenge faced by physicians when deciding whether to screen COPD patients is the potential impact of comorbidities.9 “Competing causes” due to death from another condition may attenuate the benefits of LC screening in COPD patients with multiple comorbidities. In the NLST, LC only accounted for 24% of all deaths while other diseases frequently associated with COPD, such as cardiovascular disease (25%), other cancers (22%) and respiratory conditions (10%) were frequent causes of death.3 Therefore, selection screening candidates among patients with COPD should not only be based on the risk for LC but also consider the potential impact of LDCT on life expectancy.
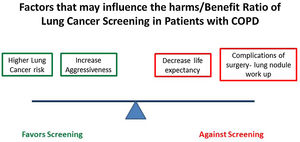
The potential implementation of LC screening in patients with COPD has not been formally proposed nor included in the current COPD guidelines. Unfortunately, available evidence on the impact of screening on LC mortality in COPD patients comes from retrospective analysis of studies not specifically designed to explore this hypothesis. A LC screening study focused on this high-risk population is urgently needed. The harms and potential benefits of a LC Screening Program in COPD patients are outlined in Fig. 1. Important questions that remain to be addressed include: Does a screening with LDCT decreases LC mortality in COPD patients? What is the role of competing risk of death in COPD patients? How we could select the best COPD candidates base on their risk? How do we balance the increased risk of LC in COPD patients with severe AO with the higher risks for surgical complications or decreases life expectancy? The potential high impact of these answers on the health of patients with COPD provide a strong rational for pursuing these studies.