Pharmacological treatment of chronic obstructive pulmonary disease (COPD) is traditionally based on bronchodilators and, in some cases, inhaled corticosteroids (ICS).1,2 This is due to the fact that the fundamental pathophysiological substrate is bronchial inflammation caused by tobacco smoke or other toxic gases, and the obstruction to airflow that this inflammation causes. However, in addition to bronchial obstruction, inflammation can cause destruction of local defense mechanisms against potentially pathogenic microorganisms (PPM).3 PPM infection can appear either acutely (exacerbations),2,4 or outside an exacerbation phase even in a situation of clinical stability (chronic bronchial infection).5
Today, international guidelines consider that the isolation (especially when repeated) of PPM in COPD even in the clinically stable phase is associated with greater severity, a greater number of exacerbations and a worse evolution of the disease.1,2Pseudomonas aeruginosa (PA) has been associated with increased mortality in COPD.6–8 However, in most series, the most frequently isolated PPM in patients with stable COPD is Haemophilus influenzae (HI).5 Despite being the most frequent PPM, little is known about its specific effect (not influenced by the isolation of other PPM) on the severity and course of COPD. Therefore, the aim of the present study is to assess the specific impact of HI on the long-term severity and course of COPD.
This is a multicenter (Requena General Hospital, Valencia and Plató Hospital. Barcelona), prospective study of 201 patients diagnosed with COPD according to GOLD guidelines, in stages II–IV. Patients were followed up exhaustively with visits every 3–6 months depending on the severity of the disease and with the systematic collection of both baseline and longitudinal data: general, exacerbations, lung function, comorbidities, severity, clinical, analytical, microbiological and therapeutic. At each medical visit, sputum samples were collected from all patients and always in the clinical stability phase (at least 4 weeks away from an exacerbation process) for microbiological assessment. Only samples with <25 squamous cells per field and more than 25 leukocytes per field were considered valid. The samples were diluted and cultured in chocolate, blood, McConkey and Saboureaud media. Sputum cultures were expressed as colony-forming units (CFU)/ml. A positive result for a particular PPM was considered to be more than 103CFU/ml (as we have used in other publications). The following were considered as PPM: H. influenzae, Streptococcus pneumoniae, Moraxella catarrhalis, Staphylococcus aureus, P. aeruginosa, Klebsiella pneumoniae, and other gram-positive and -negative bacteria. The study was approved by the ethics committee of the General University Hospital of Valencia (Spain) (ID: 2003-0089).
Two groups were established: Those patients who did not present any isolation of any PPM during the evolution or in the year prior to their inclusion in the study, and those in whom at least one HI isolation was observed throughout the study or in the year prior to their inclusion, but without the isolation of any other PPM (bacteria or mycobacteria). The comparison between both groups was established using the t-Student test or the Mann–Whitney U test depending on the distribution of the quantitative variables, and using the Chi-square test for qualitative variables. Cox multivariable regression was used to assess the independent relationship between the isolation of HI and exacerbations. A p<0.05 was considered significant.
Of the 201 patients initially included, 87 (43.3%) never had PPM isolated. Of those patients in whom PPM was isolated at some point (n=114), 57 (28.4%) had HI isolated at least once, of which 25 patients (12.4%) had only HI isolated without any other PPM being isolated during follow-up. Therefore, 112 patients were finally included for analysis (87 without PPM isolation versus 25 with HI only). None of the PPM isolates in stable phase of COPD in the patients finally included were treated with antibiotics
The mean age was 69.4 (SD: 8.2) years, with 90% being male. The mean FEV1 (% pred) was 48.7 (SD: 11.9). Most were treated with long-acting bronchodilators (91%) and 46% with ICS. The annual rate of exacerbations during follow-up was 1.4 (SD: 1.3), of which 0.46 (0.9) were severe (requiring hospitalization). The median follow-up of the patients was 82 (interquartile range: 42–102) months. The median number of valid sputum samples during follow-up was 15.1 (interquartile range: 6.9–21.1) sputum samples.
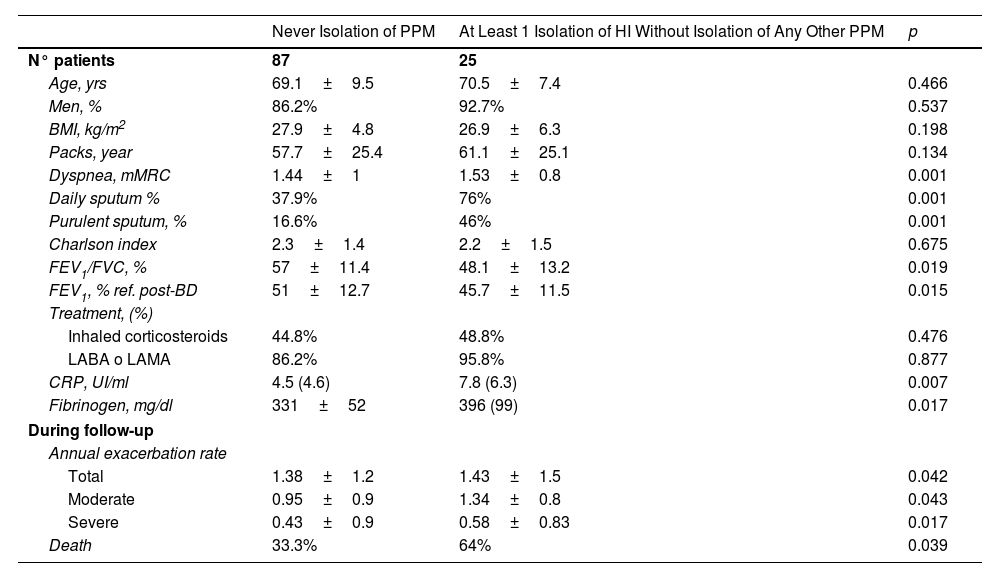
Table 1 shows that patients in whom HI was isolated at least once in one of the respiratory samples in stable phase compared to those patients in whom no PPM was ever isolated presented greater clinical severity (dyspnea and sputum quantity/purulence), greater airflow obstruction, greater systemic inflammation (according to peripheral concentration of C-reactive protein [CRP] and fibrinogen) and a higher rate of annual exacerbations. However, no differences were observed regarding age, gender, comorbidities (Charlson Index), smoking, or treatment with bronchodilators or ICS prescribed.
Comparison Between the Group of Patients Without Any Isolation of Pathogenic Microorganisms Throughout the Follow-Up With Those With Only Haemophilus influenzae Isolation.
| Never Isolation of PPM | At Least 1 Isolation of HI Without Isolation of Any Other PPM | p | |
|---|---|---|---|
| N° patients | 87 | 25 | |
| Age, yrs | 69.1±9.5 | 70.5±7.4 | 0.466 |
| Men, % | 86.2% | 92.7% | 0.537 |
| BMI, kg/m2 | 27.9±4.8 | 26.9±6.3 | 0.198 |
| Packs, year | 57.7±25.4 | 61.1±25.1 | 0.134 |
| Dyspnea, mMRC | 1.44±1 | 1.53±0.8 | 0.001 |
| Daily sputum % | 37.9% | 76% | 0.001 |
| Purulent sputum, % | 16.6% | 46% | 0.001 |
| Charlson index | 2.3±1.4 | 2.2±1.5 | 0.675 |
| FEV1/FVC, % | 57±11.4 | 48.1±13.2 | 0.019 |
| FEV1, % ref. post-BD | 51±12.7 | 45.7±11.5 | 0.015 |
| Treatment, (%) | |||
| Inhaled corticosteroids | 44.8% | 48.8% | 0.476 |
| LABA o LAMA | 86.2% | 95.8% | 0.877 |
| CRP, UI/ml | 4.5 (4.6) | 7.8 (6.3) | 0.007 |
| Fibrinogen, mg/dl | 331±52 | 396 (99) | 0.017 |
| During follow-up | |||
| Annual exacerbation rate | |||
| Total | 1.38±1.2 | 1.43±1.5 | 0.042 |
| Moderate | 0.95±0.9 | 1.34±0.8 | 0.043 |
| Severe | 0.43±0.9 | 0.58±0.83 | 0.017 |
| Death | 33.3% | 64% | 0.039 |
MPP: potentially pathogenic microorganisms; BMI: body mass index; MRC: Medical Research Council; FEV1: peak expiratory flow during the first second; FVC: forced vital capacity; Pred. predicted; CRP: C-reactive protein; LABA: long-acting beta adrenergic bronchodilators; LAMA: long-acting antimuscarinic bronchodilators.
Taken two different cut-off points (at least 1 exacerbation/year and at least 2 exacerbation/year), multivariable Cox regression adjusted by baseline dyspnea, sputum characteristics, lung function (FEV1) and CRP showed that the isolation of HI (without any other PPM) was a risk factor for both cut-off points (at least one exacerbation/year): HR 3.12 (95%CI: 1.8–6.3; p=0.003), and at least 2 exacerbation/year: HR: 2.21 (95%CI: 1.2–6.2; p=0.041).
HI is the PPM that most frequently appears infecting the airway of patients with COPD, either acutely (exacerbation) or chronically (chronic bronchial infection).5 Preliminary studies have shown that its presence is capable of generating an excess of local bronchial inflammation with the negative consequences that this circumstance causes,9 although to date we have not found any study that analyzes the consequences of HI (independent of other PPM) on the severity and evolution of the disease. According to our results, as expected, even in the stable phase of COPD, the presence (especially repeated) of HI is associated with greater severity and worse progression of the disease, including death. In fact the isolation of HI is independently related to a higher risk of annual exacerbation rate in both cut off point (at least 1 and at least 2 exacerbation/year). The main reason is probably the increase in local inflammation produced by this PPM.9 Although we do not have direct data on bronchial inflammation, we have been able to observe an increase in systemic inflammation, which in patients with COPD is usually associated with an increase in local inflammation.10 However this is a hypothesis-generating study that only can assess associations and risks but not causality. On the other hand, the intake of ICS did not influence a higher prevalence or incidence of IH isolation.11
Among the strengths of our study, it is worth highlighting that we have compared patients with HI isolates with those in whom, despite analyzing multiple samples of respiratory secretions (sputum), no PPM has been isolated at any time. This means that the results cannot be attributed to the effect of other PPM other than HI, which have been shown to contribute to this worsening of the disease, such as P. aeruginosa or other gram-negative bacteria.6–8 On the other hand, this is a very well characterized series of patients and followed up over a long period of time.
Among the limitations of our study is that only patients in stages II–IV of GOLD were included, that is, at least of moderate severity. This is because these are the patients in which PPM isolation is more frequent,5 so we could offer greater statistical power to perform the calculations (although, even with this, we cannot rule out that in some analyses there may be a type II statistical error). Therefore, our results cannot be extrapolated to patients with mild COPD. On the other hand, from a microbiological point of view, colony quantification was performed semi-quantitatively (with a cut-off point of 103CFU/ml). This was because it was the cut-off point used in other studies by our group on this same series of patients.12
In conclusion, the specific isolation of HI in patients with COPD in the clinically stable phase is associated with greater clinical severity of the disease, a higher annual rate of exacerbations, worse lung function and greater systemic inflammation. In the future, it would be important to establish microbiological studies in this regard that include analysis of changes in the lung microbiome (dysbiosis) and its consequences in terms of the damage specifically caused by HI.8 Similarly, clinical trials are needed to establish whether HI bronchial infection is a treatable trait of COPD13 (as is the case with other diseases such as bronchiectasis), either with antibiotic14 or anti-inflammatory treatment.
Conflict of InterestNone of the authors declare any conflict of interest.