Anti-TNF-α agents have revolutionized the care of inflammatory bowel disease, extending therapeutic control beyond the gut. Yet, paradoxically, they may induce sarcoid-like granulomatous reactions, a class effect increasingly reported in literature [1]. Although pulmonary and nodal manifestations have been sporadically documented [2,3], pleural disease is exceptionally uncommon. We describe a case of infliximab-induced pleural granulomatosis, underscoring its diagnostic challenge and the need for awareness of this paradoxical reaction.
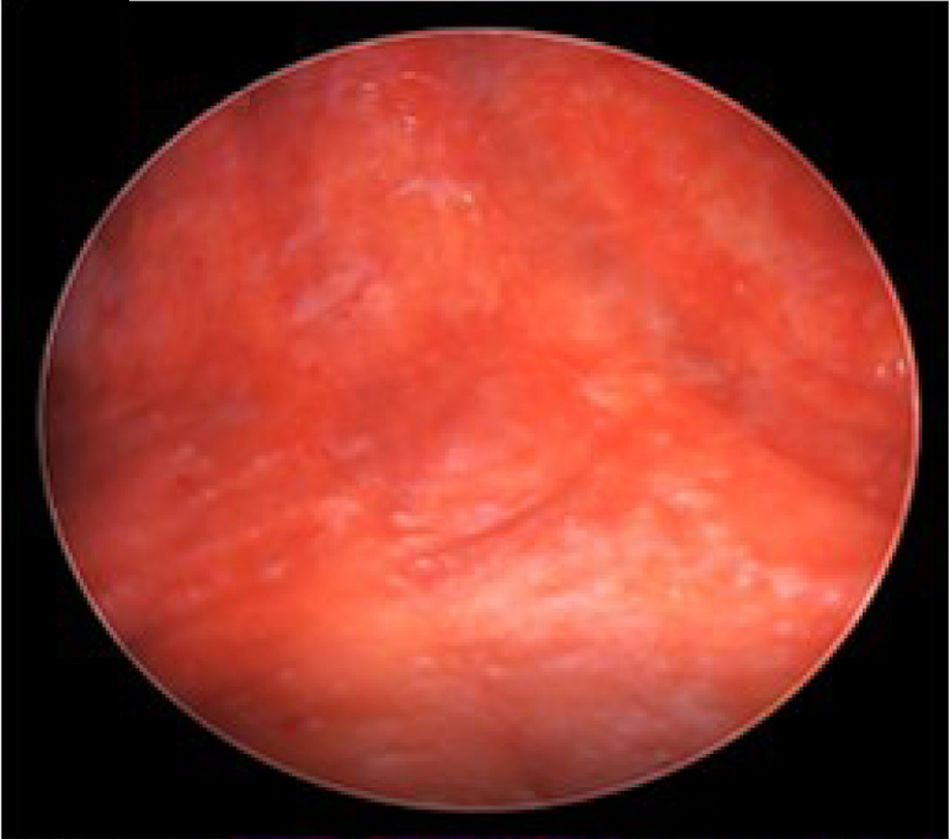
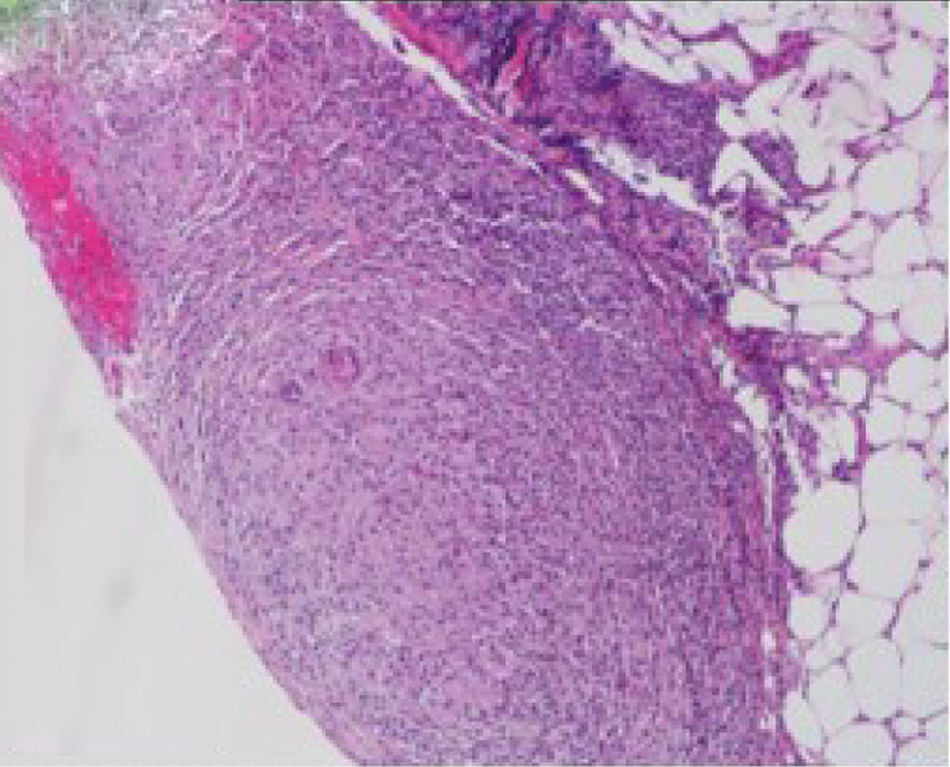
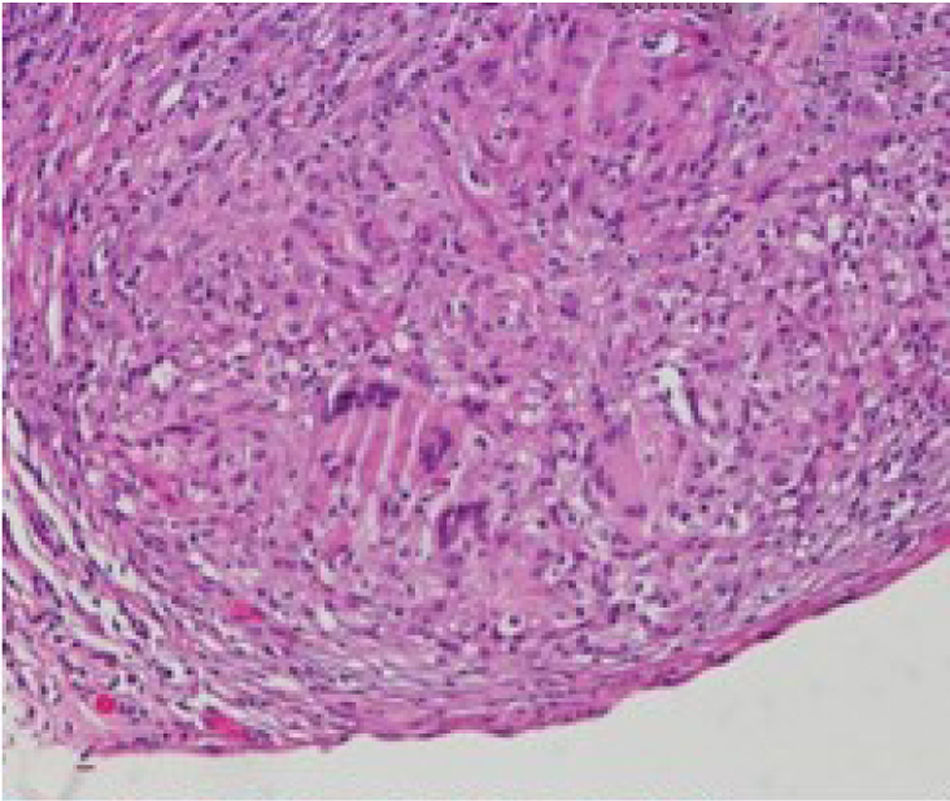
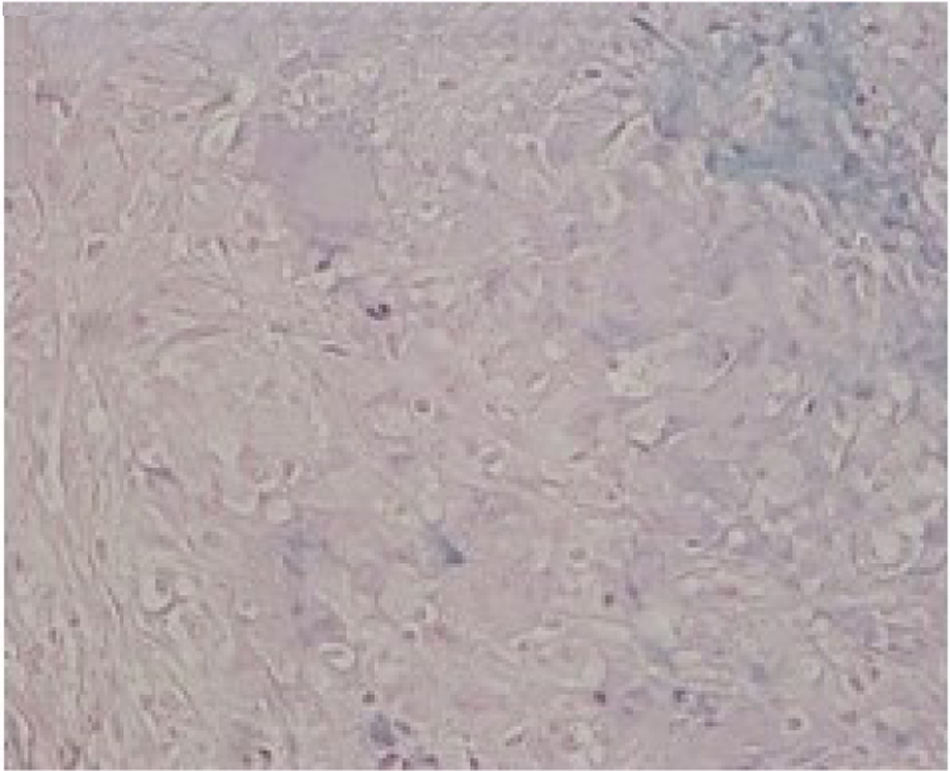
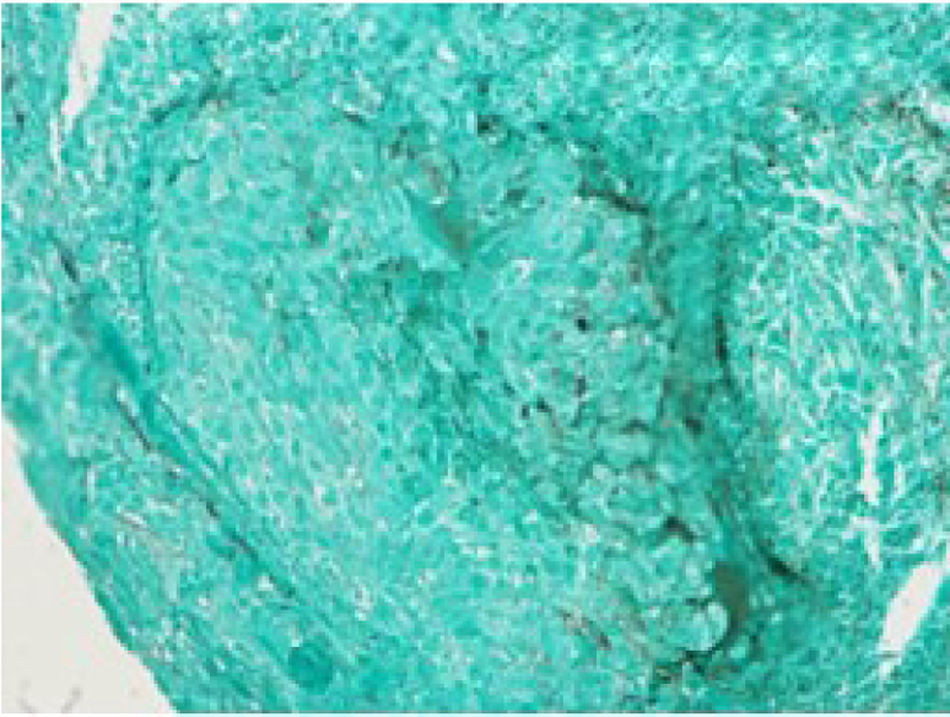
We report a case of a 50-year-old Hispanic male with a longstanding history of Crohn's disease, receiving infliximab therapy for nearly one year, At the time of symptom onset, he was receiving infliximab 5mg/kg every eight weeks with adequate disease control. He presented with progressive shortness of breath and right-sided pleuritic chest pain. Chest computed tomography (CT) revealed a moderate-sized right-sided pleural effusion associated with nodular airspace opacities, without pleural thickening or distinct masses. Repeated thoracenteses yielded exudative fluid with normal glucose and pH, lymphocyte predominance, and low ADA levels; however, Extensive analyses including bacterial cultures, cytology, and a normal serum ACE and calcium results, with negative PET/CT, argued against sarcoidosis, as well as tuberculosis testing (QuantiFERON), was negative. As tuberculosis had been excluded and thoracenteses remained nondiagnostic, a blind pleural biopsy was not pursued. Given the superior diagnostic yield and the advantage of direct pleural visualization, a medical thoracoscopy was performed, revealing diffuse pleural nodularity without distinct masses or adhesions (Fig. 1). Histopathological examination of pleural biopsies demonstrated non-caseating granulomas without malignant cells and staining for acid-fast bacilli and fungi were negative (Figs. 2–5).
Given the absence of other infectious or malignant etiologies, isolated pleural granulomatosis secondary to infliximab therapy was diagnosed. Following discontinuation of infliximab and initiation of vedolizumab, the pleural effusion and symptoms resolved completely within four weeks.
This case emphasizes the rarity and diagnostic challenge of isolated pleural granulomatosis associated with TNF-α inhibitors. While pulmonary manifestations in IBD are often parenchymal [4], isolated pleural involvement is exceedingly uncommon. TNF-α inhibitors, including infliximab, are known to induce granulomatous reactions, typically resembling sarcoidosis [5] involving lymph nodes and lung parenchyma. In our patient, Causality assessment using the Naranjo scale indicated a probable association (score 7/13). Only two cases of infliximab-induced pleural granulomatosis have been reported previously. Unlike Ali et al., who described bilateral effusions, and Yang & Dowling, who reported concomitant parenchymal disease, our case demonstrated isolated unilateral pleural involvement, as the minor nodular opacities resolved after infliximab withdrawal. The use of thoracoscopy in our patient provided direct visualization and robust histopathological confirmation, and resolution was achieved after switching from infliximab to vedolizumab. Approach to suspected infliximab-induced granulomatosis requires individualization. While early drug withdrawal may be contemplated in some circumstances, particularly when thoracoscopy is unavailable, invasive confirmation remains important when uncertainty persists; in our case, discontinuation was undertaken only after thoracoscopic diagnosis.
This report highlights this rare presentation and underscores the need to consider TNF-α inhibitor induced pleural granulomatosis in unexplained pleural effusions with granulomatous inflammation. Early recognition and drug withdrawal are essential for resolution and to avoid unnecessary interventions.
Author contributionsAyman Salih conceptualized the case, collected clinical data, and drafted the manuscript.
Rami Ahmed contributed to the literature review, diagnostic reasoning, and manuscript revision.
Mohamed Omballi provided case supervision, contributed to imaging interpretation, and critically revised the manuscript for intellectual content.
All authors reviewed and approved the final version of the manuscript.
Patient consent statementWritten informed consent was obtained from the patient for publication of this case report and the accompanying images.
Artificial intelligence involvementArtificial intelligence tools were used to assist with language enhancement and readability improvements. No content was generated or interpreted by AI.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflicts of interestThe authors declare no conflicts of interest.