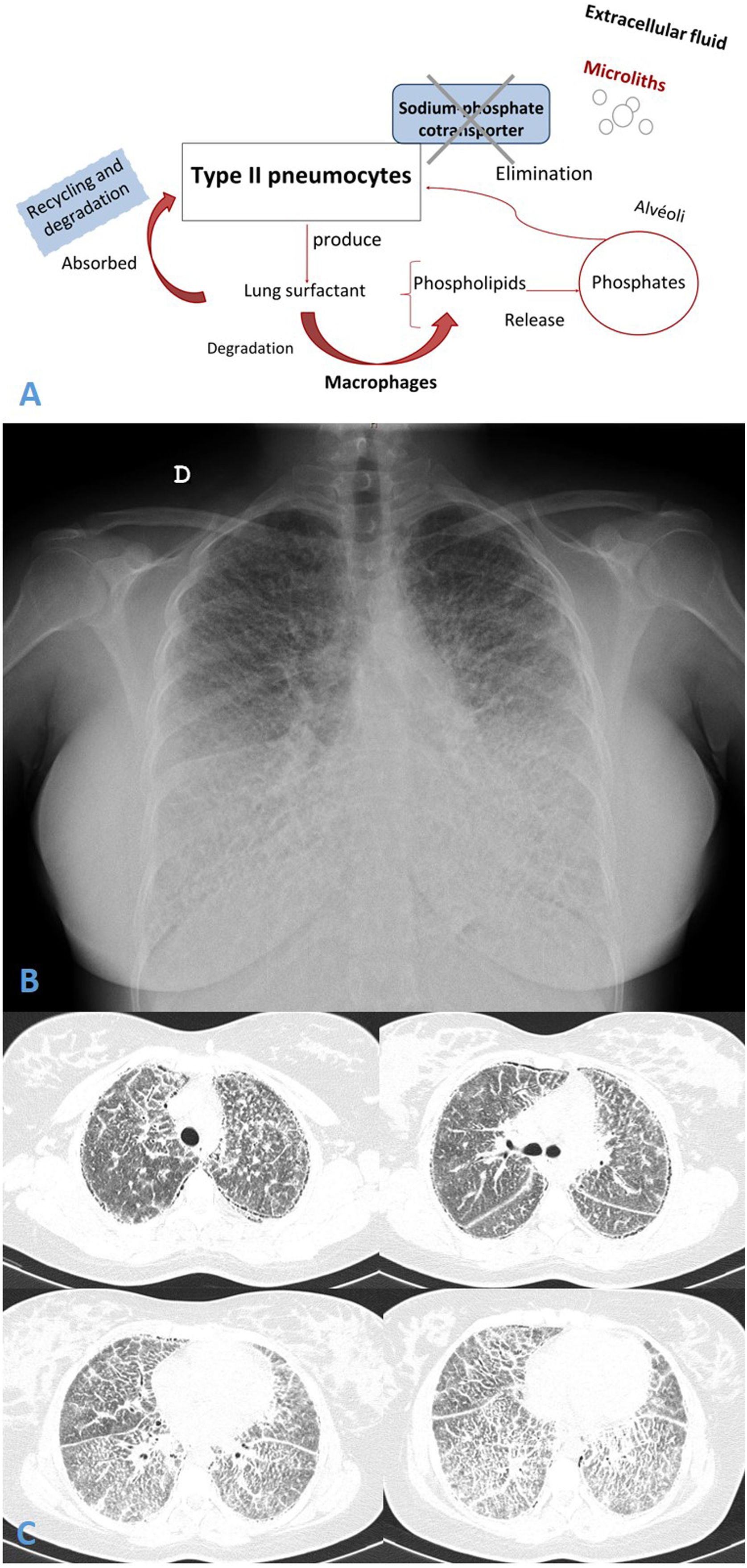
Concomitant calcium metabolism disorder is scarcely described in patients with PAM.1 It is well known that PAM is an infrequent autosomal recessive lung disease, caused by a homozygous mutation in SLC34A2 gene (chromosome 4p15). This gene encodes a type IIb sodium-dependent phosphate cotransporter (NaPi-IIb)2 playing an active role in phosphate homeostasis in several organs. SLC34A2 is primarily expressed in type II pneumocytes (AECII), but also in ileal epithelial, among others, and less in kidneys. This cotransporter dysfunction causes the AECII inability to clean intra-alveolar phosphorus ions and calcium salts precipitation developing microliths in the extracellular fluid3 (Fig. 1A).
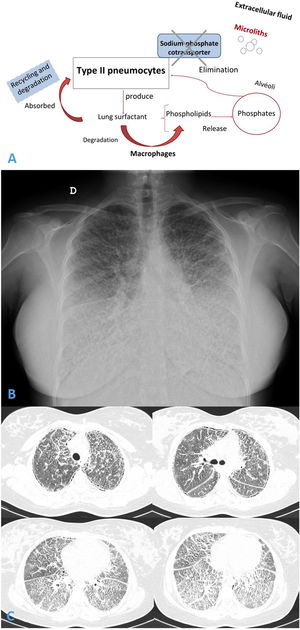
(A and B) Chest radiograph presented diffuse, fine bilateral reticulation with typical image of micronodular appearance (“sandstorm lung”). (C) Thorax high resolution computed tomography (HRCT) revealed widespread bilateral lung micro-calcifications, ground-glass opacities, interlobular septal thickening and subpleural cysts.
We present a case of PAM with SLC34A2 gene mutation and hypothetical alteration in the intestinal–renal–calcium axis.
She was a 27-year-old Moroccan female, living in Barcelona for 12 years. She had lumbar pain the year after pregnancy. Lumbar spine X-ray showed interstitial pattern in lung bases. In the anamnesis, she admitted progressive exertional dyspnea and dry cough for the last 5 years. Consanguinity between her parents (first cousins) was detected.
Physical examination revealed bibasal dry crackles, 98% oxygen saturation at rest and clubbing. Pulmonary function tests showed a moderate restrictive pattern: FEV1/FVC ratio 99%, FVC 72% (2.52L), TLC 66% (3.14L), RV 48% (0.64L), and moderate alteration of DLCO 55%. Six-minute walking test exhibited 6% of desaturation (final SpO2 92%) walking 415m without stopping, with a post-walking Borg dyspnea score of 4. Blood analysis showed negative autoimmunity, elevated PTH, decreased 1,25-dihydroxy vitamin D3 (calcitriol) levels, hypocalcemia and low fractional urinary calcium and phosphate excretion.
Chest radiograph and Thorax HRCT showed micronodular pattern (Fig. 1B and C). Microliths were identified in the bronchoalveolar lavage. Genetic study revealed pathogenic variant c.1328delT (p.Leu443Argfs*6) in the SLC34A2 gene.
It is well known that PAM is an infrequent deadly genetic disease, diagnosed before age 50, slightly predominant in men worldwide, except in Spain, Italy and France.4 Although the frequency of the mutant gene in the general population is <0.008, familial frequency occurrence ranges between 32 and 61%, usually with horizontal transmission. Consanguinity was always present, when vertical transmission was reported.3
As well as Olauson et al., we also suggest a polygenic disorder. Explaining hypocalcemia, serum elevation of PTH and hypocalciuria, because of decreased calcitriol; while hypophosphaturia could be explained by mutation of fibroblast growth factor 23 (FGF23) gene.1 We hypothesized, that there is no evidence of hyperphosphatemia due to decreased absorption of phosphate in gut by the NaPi-IIb cotransporter dysfunction and renal homeostasis.5
Therefore, is PAM associated to calcium metabolism disorder a polygenic disease? The altered function of NaPi-IIb cotransporter associated with SLC34A2 gene mutation in patients with PAM could be related to impaired calcium-phosphate metabolism. More studies are necessary to better study this axis and find effective treatments related to SLC34A2 gene or others, which could reverse the deposit of microliths in the lung and improve phosphate metabolism too. Furthermore, facing a consanguinity family history, the genetic study can corroborate the presence of the mutated gene and discard other affected relatives, in addition to carriers of the disease.
Author's ContributionsYPG: Manuscript preparation, manuscript review.
PL: Diagnosed the patient radiologically. Manuscript review.
EC: Visit patient. Manuscript review.
VVZ: Visit and control the patient. Manuscript preparation, manuscript review.
All the authors have reviewed and approved the submitted version.
FundingThe present case report has no funding.
Conflicts of InterestThe authors have no conflicts of interest to declare.
We are grateful to Dr. Núria Llecha and Dr. Ariadna Padró Miquel from the Molecular Genetic Service of the South Metropolitan Territorial Clinical Laboratory for carrying out the genetic study of our patient.