35-Year old man, with a history of bilateral lung transplant in 2018 (6 years ago) due to cystic fibrosis was admitted to our hospital with complaints of prolonged intermittent fever, dyspepsia and diarrhea lasting for one month. At the time of admission, maintenance immunosuppression consisted of 3mg of tacrolimus every 12h, 1.75mg of everolimus every 12h and 7.5mg of prednisone every 24h, with adequate levels of all the drugs mentioned. The patient had not been exposed to any contacts that could justify the development of an opportunistic disease.
Systemic examination did not reveal any significant findings other than unquantified weight loss due to decreased appetite. Blood investigations revealed normocytic hypochromic anemia with a hemoglobin level of 10.3g/dl and a total leukocyte count of 0.79×10^3/μl. C-reactive protein levels were elevated (31ng/ml) with normal procalcitonin.
Blood cultures, serologies, chest radiograph, Mantoux test, and echocardiography were unremarkable, except for a positive Quantiferon result, with a negative Mantoux test due to immunosuppression. Quantiferon had not been performed previously, and the patient had never received the BCG vaccine.
Upon admission, a diagnostic bronchoscopy was performed, which showed scarce secretions without alterations in the bronchial architecture or mucosa. Bronchoalveolar lavage was performed and referred to microbiology without any microbiological isolation, and to cytology without notable findings. Additionally, a transbronchial biopsy was performed, which ruled out rejection.
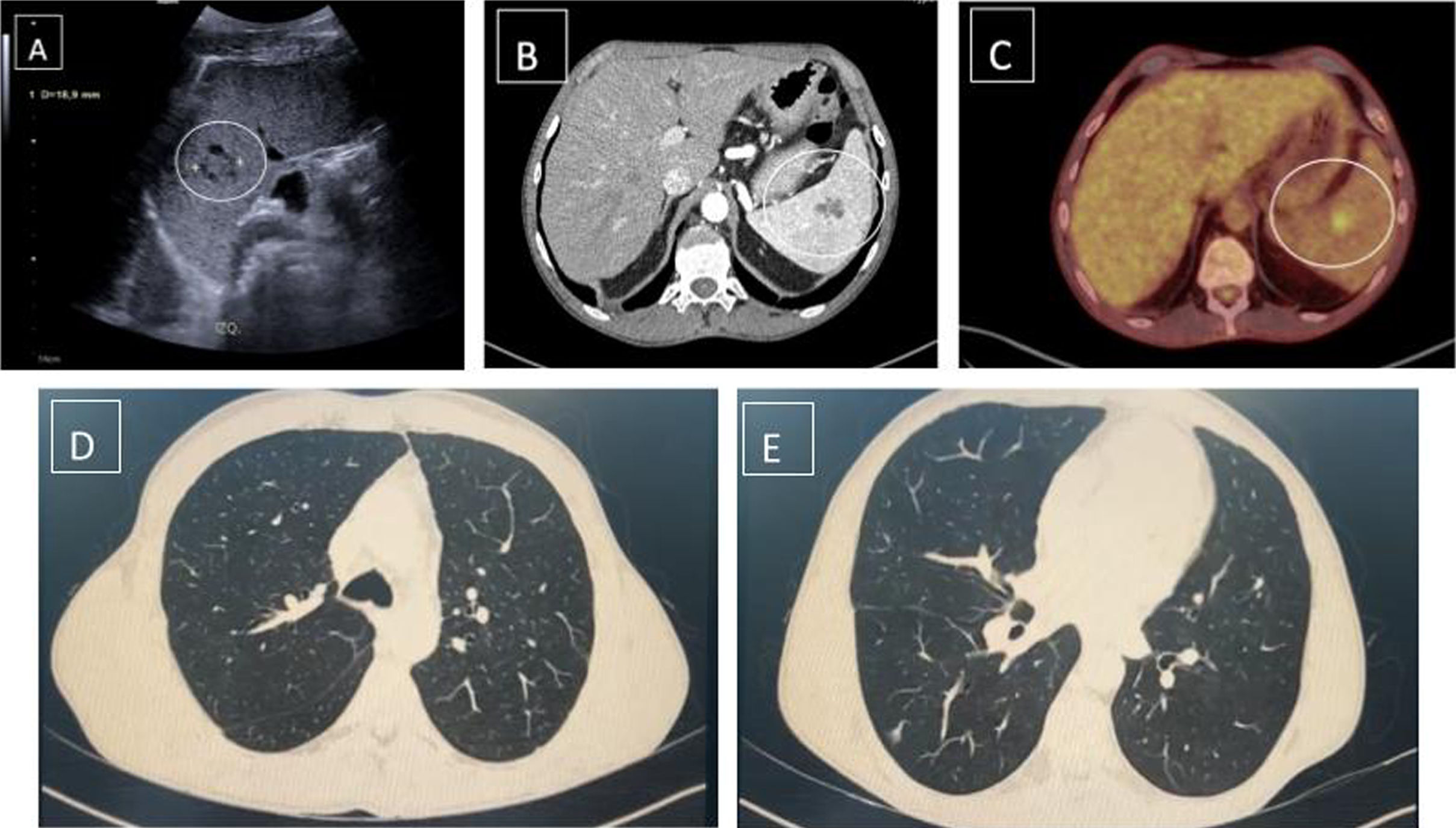
Contrast-enhanced CT (Fig. 1B) revealed a larger spleen compared to previous CT scans, identifying multiple millimetric focal lesions and a dominant, heterogeneous focal lesion of about 3cm, compatible with an abscess. Following the CT result and clinical suspicion, broad-spectrum antibiotic therapy was initiated without clear resolution of the fever and persistence of the image on follow-up CT and abdominal ultrasound (Fig. 1A). Consequently, MRI and total body PET-CT were requested to rule out malignancy.
PET-CT (Fig. 1C) revealed a known splenic lesion with a pathological increase in uptake (SUV max 4.9; healthy parenchyma 2.7), mild uptake in the maxillary sinuses, ethmoid cells, and nasal fossa, as well as opacity of the right lower lobe and prostate, showing mild inflammatory activity.
The lung image described in the PET-CT, with intermediate inflammatory activity, was not visualized in the chest CT (Fig. 1D, E) and was not compatible with pulmonary involvement due to tuberculosis. Additionally, the patient had a bronchoscopy with negative results and did not present respiratory symptoms indicative of tuberculosis disease.
Following these findings, a diagnostic needle aspiration was performed, yielding around 10ml of pus. The direct smear for acid-fast bacteria was negative, but the 60-day culture was positive for Mycobacterium tuberculosis complex after 30 days.
Drug susceptibility tests were performed on the MTB, which was sensitive. The patient was started on TB induction therapy with rifabutin (to avoid interaction between rifampin and tacrolimus/everolimus), isoniazid, pyrazinamide, and ethambutol. The patient received 2 months of 4-drug induction treatment followed by 6 months of rifabutin and isoniazid for a total of 10 months of treatment. No side effects were observed, and the patient complied with the treatment, resulting in the resolution of intermittent fever and disappearance of the splenic abscess.
Splenic tuberculosis is extremely rare. Approximately 15–20% of all tuberculosis cases are extrapulmonary; of these, 3–11% are abdominal. Splenic tuberculosis is very unusual, with only a few case reports in the literature internationally.1. Usually, the spleen is involved in disseminated TB, and primary splenic TB extremely uncommon.2
Solid organ transplant (SOT) recipients are at a 20–74 times higher risk for active TB than the general population. SOT recipients have a higher risk of death, graft dysfunction, and often face therapy limitations due to hepatotoxicity and drug–drug interactions. Disseminated infection is associated with higher mortality and occurs in 22–39% of transplant recipients with active TB.3
Splenic abscess is mostly seen in patients with an underlying immunocompromised state, including diabetes, transplant recipients, intravenous drug abuse, and HIV positivity.1
Immunodeficiency is an important risk factor for the development of splenic tubercular abscess.2
Splenic tuberculosis presents with diverse clinical symptoms. The most common symptoms are fever (82.3%), fatigue and weight loss (44.12%), and splenomegaly (13.2–100%). Therefore, it is likely to be misdiagnosed as carcinoma of the spleen, metastatic tumor, lymphoma, hemangioma, or splenic abscess. The misdiagnosis rate is high if there is no history of tuberculosis in other organs.1
Splenic tuberculosis is comparatively difficult to diagnose due to its indistinct, non-specific, and atypical clinical presentations. Tissue samples for diagnosis are usually difficult to obtain due to the invasive nature of the procedures. Conventional diagnostic methods have poor chances of recovery and are frequently deferred. Radiological findings cannot reveal the exact etiology, as they may mimic malignancy or fungal infection, and treatment delays may end up being fatal.4
Diagnosing active TB in patients with SOT is challenging due to often unusual and non-specific symptoms, leading to a substantial delay in diagnosis compared to the general population. The most common symptom of active TB in SOT recipients is fever, as shown in this case, but cough and weight loss are also seen.3
Treatment consists of antitubercular therapy and spleen-saving surgery, such as percutaneous single-time aspiration. This case is presented due to the rarity of primary splenic TB and its successful management with single-time splenic aspiration and antitubercular drugs.1
Conflict of interestsThe authors state that they have no conflict of interests.