Positive expiratory pressure (PEP) as airway clearance technique prevents airway closure during expiration, reduces gas trapping in the lung,1 increases collateral ventilation2 and improves spatial ventilation distribution.3 The addition of oscillations promotes mucus mobilization based on the reduced mucus viscosity4 and distributed flows in more airways.5 Oscillating PEP devices have been proposed in cystic fibrosis.6 Simultaneous use of PEP and nebulization is sometimes performed in cystic fibrosis patients even if its clinical benefits are still not well established.
As the drug amount reaching the lungs is essential for the clinical response of drug with a dose-dependent effect, this study aims at evaluating the effect of a new oscillating PEP device combined to a nebulizer on the drug delivery to the lungs.
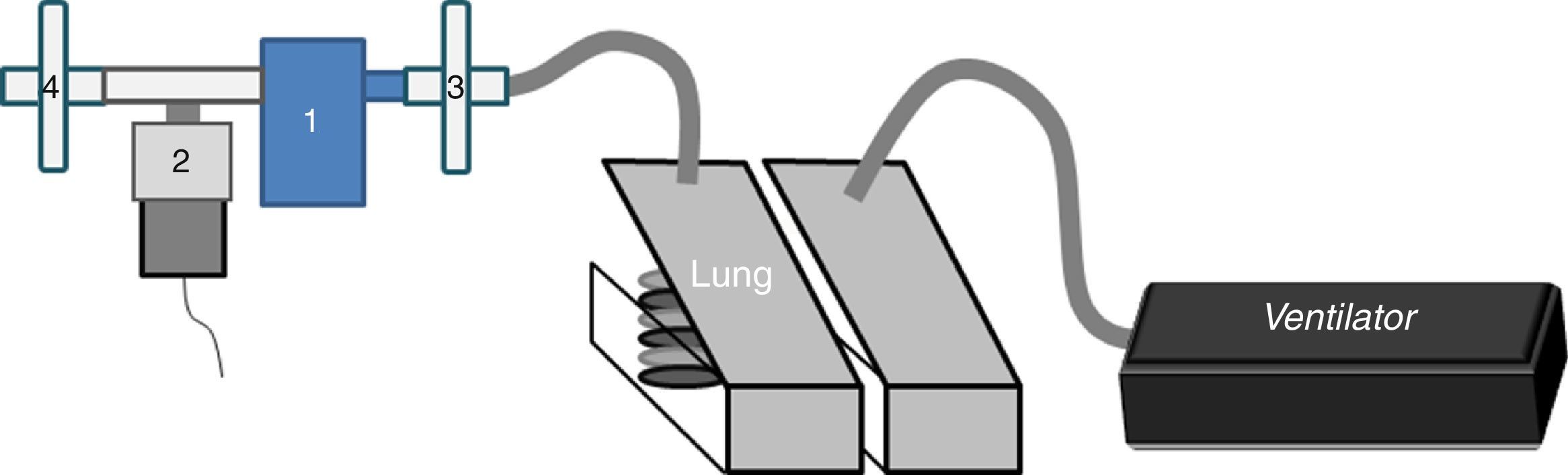
We connected a jet nebulizer (Sidestream®, Philips-Respironics) (NEB) at the distal end of an oscillating PEP device (Aerobika®, Trudell-Medical) (NEB-OPEP) with a T-piece blocking the extra vent. Each nebulizer was filled with 250mg of amikacin diluted with 3mL of normal saline.
In the in vitro part, a dual-chamber test lung (5600i Dual Adult Test Lung; Michigan Instrument Inc.) driven by a ventilator (Servo-i®, MAQUET) (Fig. 1) simulated an adult breathing pattern (Vt=500mL-RF=12breaths/min-I/E ratio=1:2-breath-hold time=0.25s). An absolute bacterial/viral filter (Air Safety Ltd, Lancashire) was placed between the lung model and NEB-OPEP/NEB to measure the inhaled dose (IND). Another filter placed on the expiratory port collected the exhaled dose (ExD). The mass of amikacin collected on filters during nebulization was quantified using the residual gravimetric method.7 The nebulizer output was calculated by dividing the IND by the nebulization duration. The residual volume was quantified.
In the in vivo part, after ethical approval (B40320107908) and registration of the trial (NCT02535130), six non-smoker healthy males were recruited. They signed a written informed consent form. They were excluded if they received any antibiotic or aerosolized drug during the month preceding the experiments, for history of cardiovascular and/or pulmonary disease, for allergy to aminoglycosides and for abnormal pulmonary function.
After (1) selection visit, spirometry and medical examination and (2) training to inhale correctly, (3) inhalation and (4) urine sampling were performed. Each subject repeated the steps 3 and 4 in similar conditions using a randomized crossover setting (www.randomizer.org) with a one-week washout period between the two configurations (NEB or NEB-OPEP). The subjects breathed spontaneously through mouthpiece wearing a noseclip.
Just before the experiments, the urinary bladder of the subjects was emptied. Then, urine samples were collected at each spontaneous micturition during the 24h following the nebulization. The volume and timing of micturition were recorded.
After sampling by fluorescence polarization immuno-assay, the total daily amount of amikacin excreted in the urine (Cu max) reflecting lung deposition and the elimination rate constant was calculated using the Michaelis–Menten kinetic model from cumulating amikacin amount measured at each micturition (Cu) and represents the lung dose (LD) (Cu max=Cu×(1−e−Ke·x·t)).
The residual amount of drug was calculated by multiplying residual volume and its final concentration.
In vitro, no difference was found between NEB and NEB-OPEP for IND (34.4% (30.0–38.0) vs 29.6% (26.8–32.0)) and ExD (20.8 (19.6–23.2) vs 25.2% (22.4–28.4)). Nebulizer output was higher for NEB than for NEB-OPEP (1.49±0.14 vs 1.10±0.12mLmin−1; p=0.032).
Six subjects completed the study (21.8±1.0 y – 179.2±8.8cm – 76.5±11.7kg – FEV1: 97±7%). The lung dose was reduced by 40% and the time required to finish the nebulization was 1.2min longer with NEB-OPEP than with NEB (Table 1).
Lung delivery comparison between nebulization used alone and coupled with an oscillating positive expiratory pressure device in 6 healthy subjects.
| NEB | NEB-OPEP | p value | |
|---|---|---|---|
| Lung dose (% ND) | 4.7±1.4 | 2.8±0.2 | 0.029* |
| Time of nebulization (min) | 4.3±0.7 | 5.5±0.6 | 0.012* |
| Residual volume (mL) | 0.85±0.08 | 1.04±0.12 | 0.044* |
Results are expressed as mean±SD. NEB: nebulizer; NEB-OPEP: nebulizer couple with an oscillating positive expiratory pressure device; ND: nominal dose.
Lung delivery around 5% of the ND with NEB confirmed previous results.8 In addition, our results showed that interposing this new OPEP device between the nebulizer and the patient's mouth reduced the efficiency of the nebulization similarly to results found with other PEP devices under different conditions.9,10 This is clinically important when dose-dependent drugs are administered. The reduced lung delivery could be explained by the impaction of particles in the PEP device that filters the larger particles.9,11 However, this hypothesis was neither verified in our study (similar IND and ExD) nor in a previous study.12
The duration of the nebulization was inversely related to the adherence in CF13 and it is therefore considered as a key factor for an optimal treatment.14,15 In the present study, the small increase in time (+1.2min) related to the NEB-OPEP is compensated by the time saved on the total duration of the treatment administered separately (3.1min). This advantage could be important for the patients needing several daily sessions. However, as suggested by our results, the necessity to nearly double the nebulization duration to reach a similar lung delivery with NEB-OPEP must be taken into account. As Berlinsky showed variable delivery depending on the PEP devices,11 each configuration must be evaluated.
The modified pattern of breathing related to PEP is unfavorable to the nebulization function and explains probably the worst lung delivery related to the combination. The reduction in breathing frequency (7.8 cycles by minutes) when healthy subjects used PEP mask could be considered as advantageous16 but it was associated to a prolonged expiration that contributes to loose particles during the expiratory phase.17 The concomitant reduction in the inspiratory–expiratory ratio contributes certainly to the reduced lung delivery by losing particles during the expiratory phase and it can explain the difference with in vitro results. Some particles are probably exhaled and others are delivered in the atmosphere or the device during this phase.
Some limitations of the study should be discussed. First, the nebulizer was placed at the distal outlet of the PEP device. This configuration is less efficient than that in which the proximal outlet is used for connection,10 although the latter is recommended by the manufacturer. Second, testing healthy subjects omits the clinical and pathophysiological outcomes related to positive expiratory pressure and its oscillations but it permits to evaluate the nebulization function8,18,19 and reduces the variability resulting from mucus plugging or lung disease. Furthermore, even if lung delivery in healthy subjects cannot be extrapolated to patients with respiratory diseases, a similar lung deposition was recently found between healthy subjects and patients with CF.20 Third, each subject was its own control to reduce anatomical, anthropometrical and respiratory influences on lung delivery.
This study suggests that connecting Aerobika at the proximal outlet of a nebulizer is less efficient than using the nebulization alone for drug delivery. However, the gain in time is an argument in favor of the combination.
We gratefully acknowledge Julien Anquetil for his help to collect data.