Since December 2019, the ongoing pandemic of coronavirus disease 2019 (COVID-19), with the culprit pathogen of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has resulted in a substantial socioeconomic burden globally. Concerns have been raised to identify the factors contributing to the development of severe or critical illness, which may help early triage and improve the clinical outcomes of COVID-19.1
Comorbidities have been common and correlate significantly with the risk of reaching to the composite clinical outcomes [requiring invasive or non-invasive ventilatory support, intensive care unit (ICU) admission or death] of patients with COVID-19.2,3 Because the respiratory tract is the first portal of viral infections and because chronic respiratory diseases (CRDs) are prevalent globally, then it comes to the next important clinical question – how CRDs impact on the clinical outcomes of COVID-19?
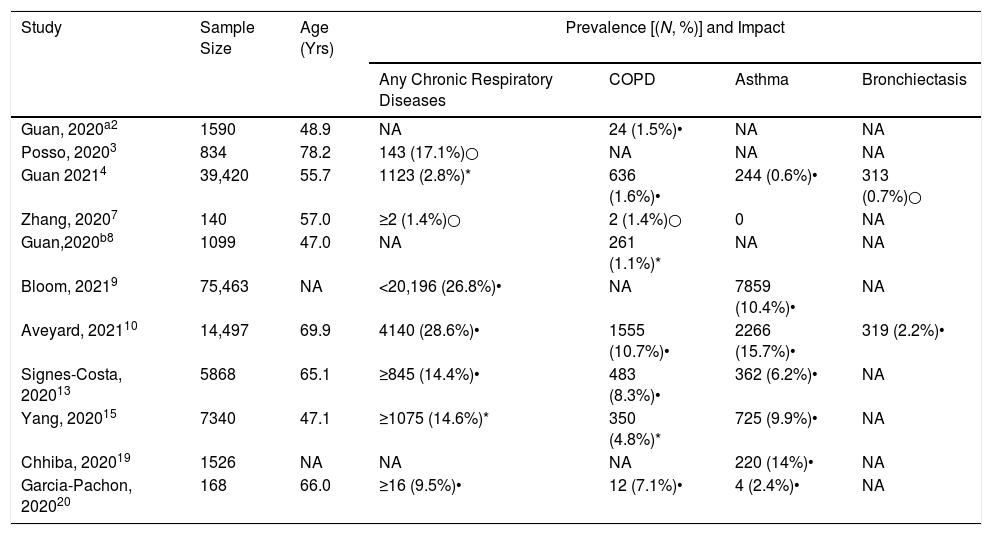
Findings pertaining to the prevalence of CRDs among patients with COVID-19 remained controversial (Table 1). A large retrospective cohort study based on the Chinese national database including 39,420 cases demonstrated a prevalence of 1.6% for chronic obstructive pulmonary disease (COPD) and 0.6% for asthma,4 which was markedly lower than that of the Chinese general population (8.6% and 4.2%, respectively).5,6 The under-representation of the CRDs was in line with earlier reports from China.2,7,8 By contrast, results from the International Severe Acute Respiratory and emerging Infection Consortium (ISARIC) World Health Organization Clinical Characterisation Protocol UK study9 reported a higher proportion of patients with asthma (10.4%) and non-asthmatic chronic pulmonary diseases (18%) among patients with COVID-19 as compared with the general population. Some factors might have contributed to the notable difference among these observational studies. First, there could have been some bias of self-reporting (under-reporting). In addition, the missing information and a lack of documentation of CRDs in the clinical charts as an important source of data collection cannot be precluded particularly given the burnout of clinicians at the time of a surge in cases and the lack of a nationwide standardized electronic data registry system. Furthermore, to avoid cross-contamination, lung functions test could not be performed in many regions of China, which might have led to the under-diagnosis of COPD and asthma. Therefore, direct comparisons of the prevalence of CRDs cannot be made in patients with COVID-19 globally (Table 1).
The Prevalence of Chronic Respiratory Diseases in Patients With COVID-19.
| Study | Sample Size | Age (Yrs) | Prevalence [(N, %)] and Impact | |||
|---|---|---|---|---|---|---|
| Any Chronic Respiratory Diseases | COPD | Asthma | Bronchiectasis | |||
| Guan, 2020a2 | 1590 | 48.9 | NA | 24 (1.5%)• | NA | NA |
| Posso, 20203 | 834 | 78.2 | 143 (17.1%)○ | NA | NA | NA |
| Guan 20214 | 39,420 | 55.7 | 1123 (2.8%)* | 636 (1.6%)• | 244 (0.6%)• | 313 (0.7%)○ |
| Zhang, 20207 | 140 | 57.0 | ≥2 (1.4%)○ | 2 (1.4%)○ | 0 | NA |
| Guan,2020b8 | 1099 | 47.0 | NA | 261 (1.1%)* | NA | NA |
| Bloom, 20219 | 75,463 | NA | <20,196 (26.8%)• | NA | 7859 (10.4%)• | NA |
| Aveyard, 202110 | 14,497 | 69.9 | 4140 (28.6%)• | 1555 (10.7%)• | 2266 (15.7%)• | 319 (2.2%)• |
| Signes-Costa, 202013 | 5868 | 65.1 | ≥845 (14.4%)• | 483 (8.3%)• | 362 (6.2%)• | NA |
| Yang, 202015 | 7340 | 47.1 | ≥1075 (14.6%)* | 350 (4.8%)* | 725 (9.9%)• | NA |
| Chhiba, 202019 | 1526 | NA | NA | NA | 220 (14%)• | NA |
| Garcia-Pachon, 202020 | 168 | 66.0 | ≥16 (9.5%)• | 12 (7.1%)• | 4 (2.4%)• | NA |
Shown with the cells are the absolute number and the percentage. Age is expressed as the mean or median. Chronic respiratory disease: having at any of COPD, asthma and bronchiectasis. •: negative impact on the outcome of COVID-19; ○: no adverse impact on the outcome of COVID-19. *: unknown impact on the outcome of COVID-19; NA: not available.
The association between CRDs and the outcomes of COVID-19 also remains an appealing question. From the Chinese national database,4 patients with COPD [Odds ratio (OR): 1.71, 95% confidence interval (95%CI): 1.44–2.03] and asthma (OR: 1.45, 95%CI: 1.05–1.98) were more likely to reach the composite clinical outcomes, but not death, compared to those without. Similarly, a recent cohort study using the Qresearch database10 found that CRDs (including COPD, asthma, bronchiectasis, idiopathic pulmonary fibrosis and lung cancer) were associated with an increased risk of hospitalization and death. These studies implied poorer outcomes among who had coexisting CRDs. However, considering that CRDs consisted of different diseases with largely heterogeneous pathophysiology, and the notably variations in the study designs, a more detailed analysis is required to fully decipher the link between individual CRDs and the outcomes of COVID-19.
COPD is one of the most prevalent CRDs, with the age-standardized prevalence of 3.2% in males and 2.0% in females globally.11 COPD has consistently been a risk factor of the adverse outcomes of COVID-19.2,9,10,12,13 The high expression levels of ACE2 in the small airway epithelium of smokers and COPD patients have indicated that both COPD and smokers were at risk of developing adverse outcomes of COVID-19.12,14
However, the evidence has been less straightforward to account for the association between asthma and the outcomes of COVID-19. A study from Korea including ∼220,000 participant revealed a greater susceptibility to contracting SARS-CoV-2 among asthmatic patients (OR, 1.07; 95%CI, 1.00–1.15) and developing adverse clinical outcomes (OR, 1.62; 95%CI, 1.01–2.67) compared with those without asthma.15 The association between asthma and COVID-19 has further been complicated by the severity of asthma. For instance, the ISARIC study found that only patients with severe asthma had a significantly increased risk of death compared with those without asthma [adjusted hazards ratio, 1.96; 95%CI, 1.25–3.08].9 Findings from the OpenSafely database have also added the similar evidence.16 However, patients with mild-to-moderate asthma did not present with a significantly poorer prognosis compared to patients without asthma.9 Moreover, the use of medications might have also altered the clinical outcomes of COVID-19. The OpenSafely study demonstrated that COVID-19 patients with asthma who were prescribed with high-dose ICS were associated with an increased risk of death (adjusted hazards ratio, 1.55; 95%CI, 1.10–2.18), whereas those receiving a low or medium dose were not (aHR, 1.14; 95%CI, 0.85–1.54].17 The adverse impact of asthma could also be interpreted by the fact that respiratory viruses are a major cause of asthma exacerbations, and that asthmatic patients frequently yielded deficient innate immune responses to viral infections.18
By contrast to these negative findings, some studies reported no significant difference on SARS-CoV-2 infection, hospital admission or death between patients with asthma and those without.7,13,19,20 Furthermore, in another meta-analysis which consisted of 131 studies and 410,382 COVID-19 patients, asthmatic patients had a lower risk of death [risk ratio (RR), 0.65; 95%CI, 0.43–0.98], but not hospitalization or the receipt of mechanical ventilation, as compared with those without asthma.21 There have been some postulations regarding the protection from asthma among patients with COVID-19. Unlike COPD which is mainly characterized by type 1 airway inflammation, asthmatic patients reportedly had a decreased level of angiotensin-converting enzyme 2 (ACE2) expression due to the type 2-skewed inflammation.12 In addition, around 70%–85% of asthmatic patients were receiving ICS treatment,17 which could have attenuated the expression of ACE2 and transmembrane protease serine 2 (a host serine protease critical to spike protein priming for cell entry),22 both of which are crucial modulators of SARS-CoV-2 infection.23 For instance, an in vitro study revealed that ciclesonide suppressed SARS-CoV-2 replication.24
By contrast to asthma and COPD, findings of bronchiectasis have been less clear. From the QResearch database, bronchiectasis was a risk factor of hospitalization but not ICU admission and death among patients with COVID-19,10 whereas no clear association between bronchiectasis and the adverse outcomes of COVID-19 has been shown from the Chinese nationwide database.4 Compared with COPD, bronchiectasis is also frequently characterized by the type 1-skewed airway inflammation but the infections of other pathogens (i.e. Pseudomonas aeruginosa, Haemophilus influenzae) could be more common and prominent. Unfortunately, the latest findings cannot be interpreted by any existing knowledge although much information of the jigsaw puzzle is missing (i.e. the severity, inflammatory milieu and the spectrum of pathogens of bronchiectasis). Studies with a larger sample size and complete data sets are needed to fully ascertain these associations in patients with bronchiectasis.
CRD overlap is defined as the co-existence of two or more individual CRDs, which conferred a higher mortality rate, more frequent exacerbations and greater disease severity.25 However, no additive effect was seen in COVID-19 patients with CRD overlap as compared with those with individual CRDs.4 Therefore, no firm conclusions could be made because of the small sample sizes of the overlap group to verify the prognostic implications of CRD overlap.
The outbreak of COVID-19 has posed an enormous challenge to the scientific research on other respiratory diseases such as the CRDs whose burden has also been substantial globally. Many non-COVID-19-related clinical trials have to be ceased and many of the clinical and laboratory testings such as lung function tests and induced sputum assays were abandoned due to the concerns of cross-contamination. Some patients were not allowed or willing to complete the out-patient visits within the hospital or community health care settings. Despite all these challenges, we are reassured that COVID-19 clinical trials have been moving forward rapidly, allowing for the better evidence to inform our clinical practice. However, the impact of COVID-19 on CRDs after the convalescent stage remains an open research question.
From all available evidence, it has now become increasingly clear that COVID-19 patients with COPD and severe asthma, but not mild-to-moderate asthma, have a greater likelihood of progressing to severe illness or death. More precautions should be taken against the exposure to SARS-CoV-2 and prompt triage should be implemented upon hospital admissions once coexisting CRDs have been identified among patients with COVID-19.
Support StatementThis study is supported by Guangzhou Institute for Respiratory Health Open Project (funded by China Evergrande Group) Project No. 2020GIRHHMS09 and 2020GIRHHMS19, Zhongnanshan Medical Foundation of Guangdong Province (funding number not applicable, WJG), and Penghua Care Fund to the Medical Pioneers against COVID-19 of Shenzhen Social Commonweal Foundation (funding number not applicable, WJG).
Author ContributionsZhen-feng He and Wei-jie Guan drafted the manuscript; Wei-jie Guan and Nan-shan Zhong critically revised the manuscript. All authors have approved the final submission.
Conflicts of InterestThe authors declared no conflict of interest with any financial organization regarding the material discussed in the manuscript.
We thank Shan-shan Zha (Shenzhen People's Hospital) and Li-li Guan (Guangzhou Institute for Respiratory Health) for their valuable suggestions.