Bronchopleural fistula (BPF) is associated with high morbidity and mortality rates in patients undergoing pulmonary resections. Surgery, bronchoscopy, and conservative management have their limitations for small fistulas. Platelet-rich plasma (PRP) has regenerative properties, which might be efficient in enhancing tissue recovery and repairing small BPF. This study aimed to investigate efficacy and safety of PRP on BPF.
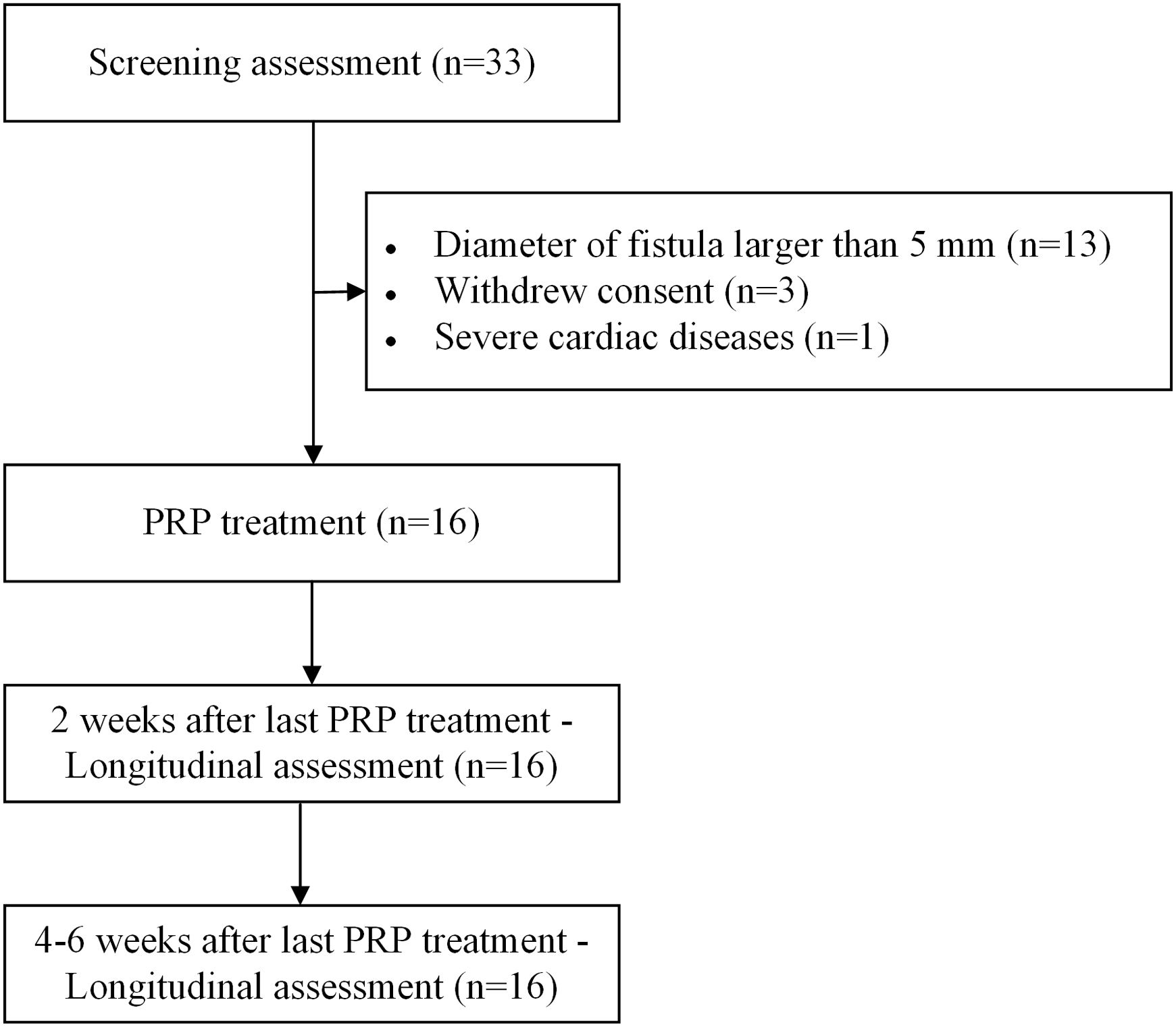
MethodsThis is a pilot prospective cohort study. Patients whose fistulas smaller than 4mm were enrolled in this study, treated with PRP under bronchoscopy and followed up at 2 weeks and 4–6 weeks after the last PRP treatment. The cure rate, improvement rate and ineffectiveness rate were investigated. The severity of respiratory symptoms was evaluated by modified Medical Research Council dyspnea scale (mMRC) and COPD Assessment Test (CAT). The recurrence of fistula, new infection and mortality rate were examined. Adverse events were documented to explore the safety profile of PRP therapy.
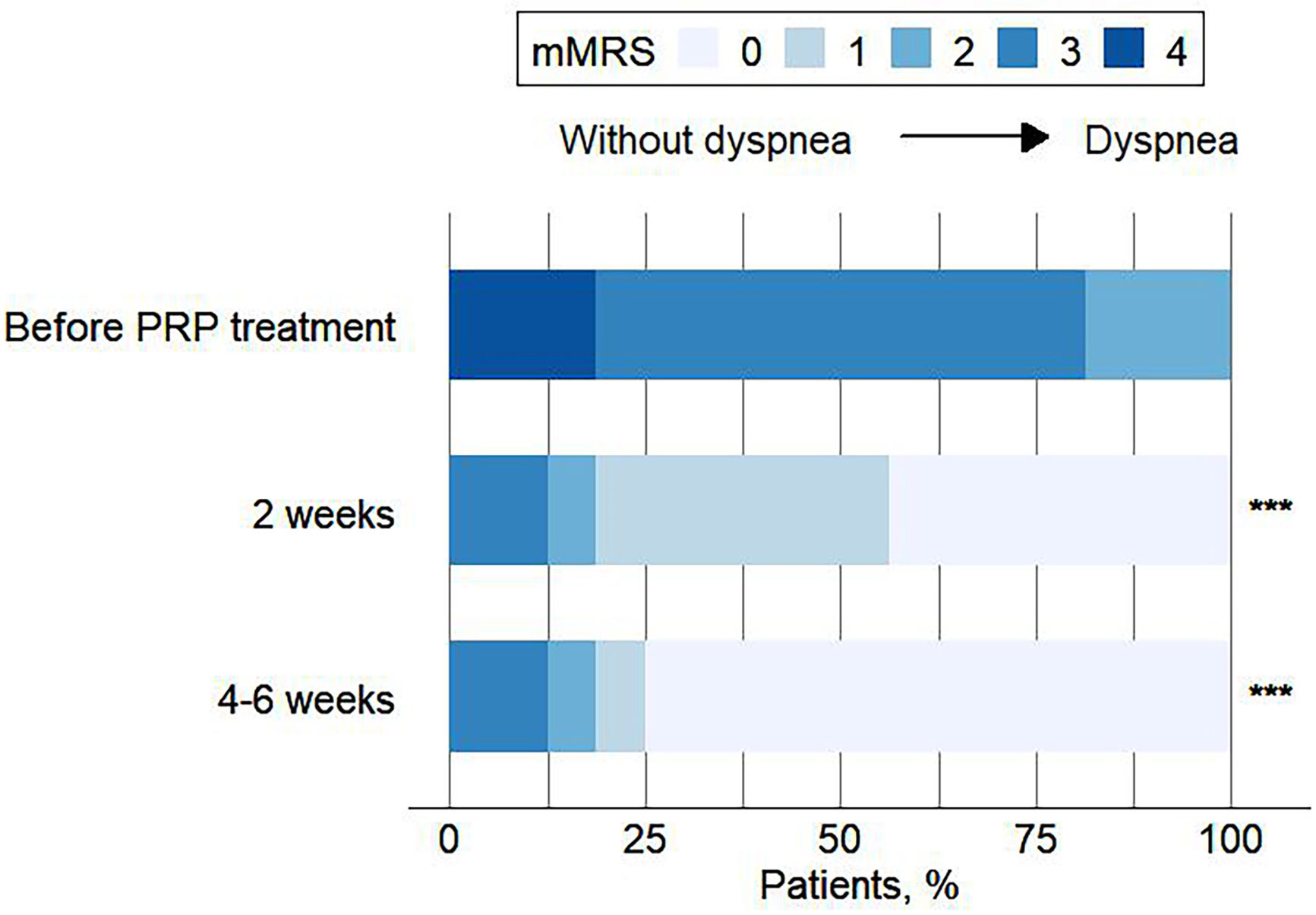
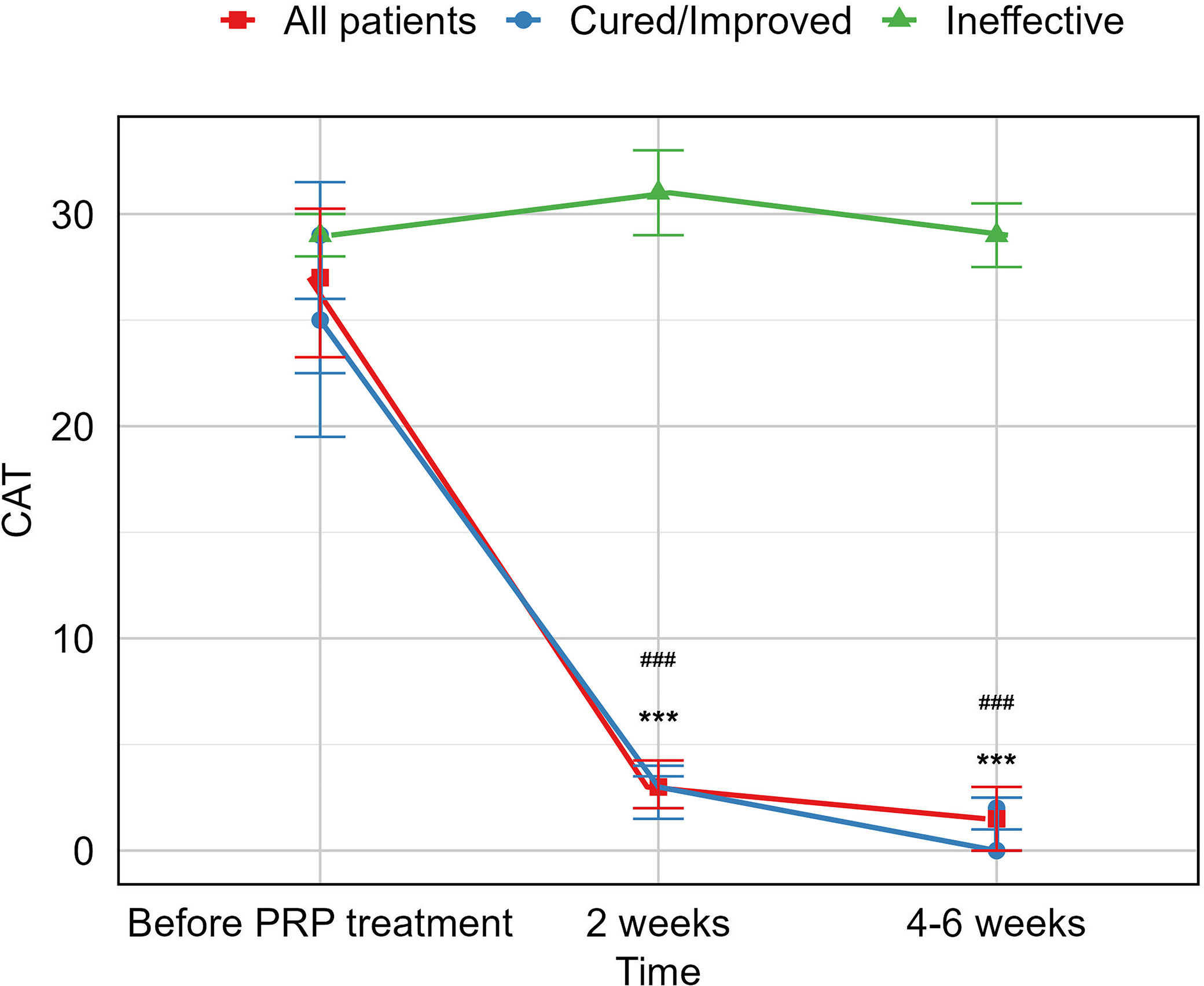
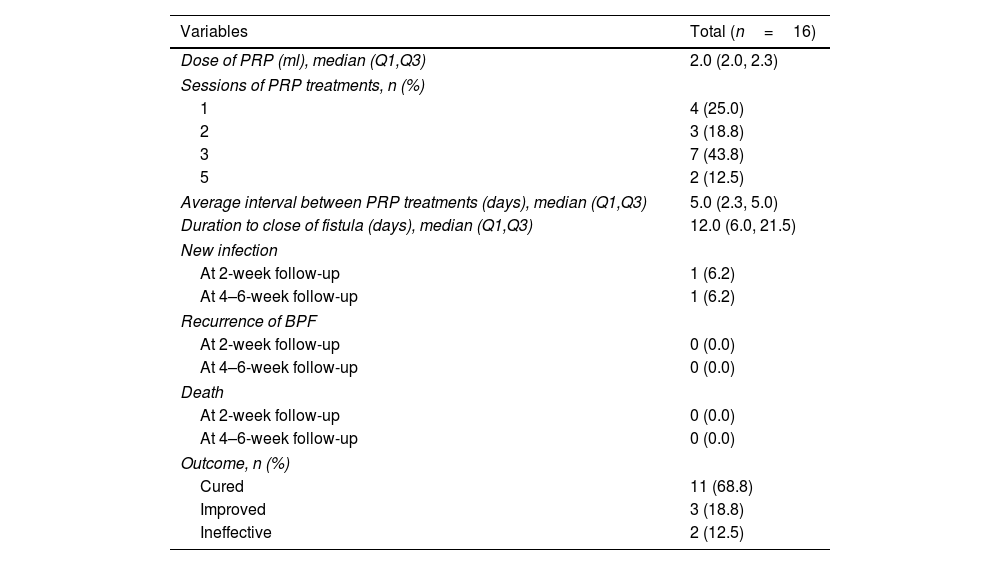
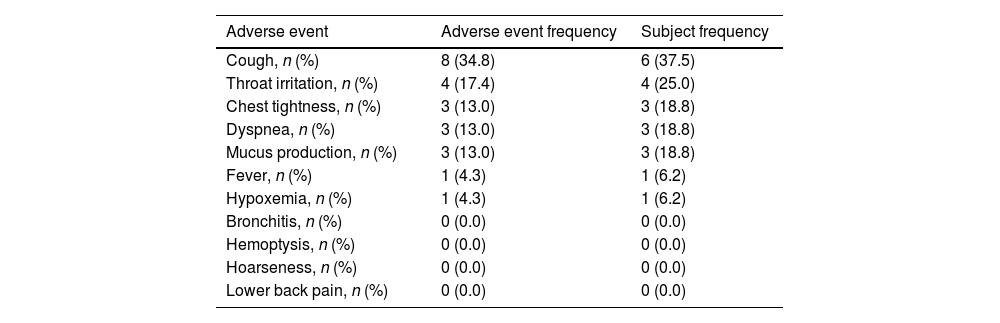
ResultsA total of 16 patients (mean age, 50.1 years) met the eligibility criteria. The median time from the first PRP treatment to the closure of the fistula was 12.0 (IQR 6.0, 21.5) days. Our findings indicate an effectiveness rate of 87.6%, with 68.8% of cure and 18.8% of improvement, along with significant improvement of respiratory symptoms evaluated by mMRC (P<0.001) and CAT (P<0.001). No recurrent of fistulas, newly developed infection, or death was observed. Adverse events of the procedure were most mild (82.6%) and temporary.
ConclusionsPRP is a potential treatment for small BPF and is well tolerated.