To compare the efficacy and safety of indwelling pleural catheters (IPC) in relation with the timing of systemic cancer therapy (SCT) (i.e., before, during, or after SCT) in patients with malignant pleural effusion (MPE).
MethodsSystematic review of randomized controlled trials (RCT), quasi-controlled trials, prospective and retrospective cohorts, and case series of over 20 patients, in which the timing of IPC insertion in relation to that of SCT was provided. Medline (via PubMed), Embase, and Cochrane Library were systematically searched from inception to January 2023. The risk of bias was assessed using the Cochrane Risk of Bias (ROB) tool for RCTs and the ROB in non-randomized studies of interventions (ROBINS-I) for non-randomized designs.
ResultsTen studies (n=2907 patients; 3066 IPCs) were included. Using SCT while the IPC was in situ decreased overall mortality, increased survival time, and improved quality-adjusted survival. Timing of SCT had no effect on the risk of IPC-related infections (2.85% overall), even in immunocompromised patients with moderate or severe neutropenia (relative risk 0.98 [95%CI: 0.93–1.03] for patients treated with the combination of IPC and SCT). The inconsistency of the results or the lack of analysis of all outcome measures in relation to the SCT/IPC timing precluded drawing solid conclusions about time to IPC removal or need of re-interventions.
ConclusionsBased on observational evidence, the efficacy and safety of IPC for MPE does not seem to vary depending on the IPC insertion timing (before, during, or after SCT). The data most likely support early IPC insertion.
Malignant pleural effusion (MPE) is a marker of advanced cancer, which should be treated symptomatically to preserve quality of life. Although some patients are initially asymptomatic and, therefore, do not require invasive procedures,1,2 most eventually develop dyspnea at exertion or rest, and their treatment should aim to alleviate symptoms in a minimally invasive manner.1 Traditionally, pleurodesis with talc has been the treatment of choice for MPE patients. Subsequently, indwelling pleural catheter (IPC) emerged as a valuable option for patients with non-expandable lung or failed pleurodesis,1,3 offering long-term symptom control following regular fluid drainage, with the same effectiveness as talc pleurodesis.4,5
Advances in pleural palliation have facilitated the widespread adoption of IPCs as a primary intervention in selected patients with symptomatic recurrent MPE.1,5 IPCs’ main advantages over talc slurry pleurodesis via chest tube include a shorter hospital stay and fewer requirements for additional pleural interventions; however, the rate of complications may be higher.2,3 Furthermore, talc may similarly be instilled through a functioning IPC to achieve pleurodesis.6 According to current guidelines, IPC is a suitable option for both patients with expandable and non-expandable lungs, which can be inserted and managed in the outpatient setting.1,5,7
Controversy still exists among oncologists and interventional pulmonologists with respect to the precise timing of the IPC placement, whether it should be placed before or during systemic cancer therapy (SCT) or even wait until SCT completion. Arguments in favor of the first option claim to avoid complications related to pleural effusions, including persistent symptoms, poor quality of life, and development of septations or non-expandable lungs. Arguments in favor of waiting for the oncologic treatment to take effect include: (1) potential risk of IPC infection during administration of some therapies, especially chemotherapy; and (2) potential efficacy of SCT at controlling MPE, thus precluding the need for an IPC. No clear guidelines on this topic exist. Current literature about the timing of IPC insertion in relation to SCT is limited, mainly consisting of small retrospective series or single-arm prospective studies. Moreover, no conclusions can currently be drawn on the usefulness of SCT in MPE control.4 According to current guidelines, definitive pleural interventions should probably not be deferred until SCT has been completed.5
Therefore, our aim was to critically review the existing literature in order to compare the efficacy and safety of IPC insertion for MPE in relation to the timing of SCT, with the IPC being evaluated before, during, or after SCT.
MethodsWe performed a systematic review (SR) based on the Cochrane Handbook guidelines of a non-Cochrane review. The PRISMA statement, flow chart, and checklist were being adhered to for the reporting.
Search strategy and selection processTwo librarians developed the search strategy using the PRESS guidelines to cross-check results.8 Medline via PubMed (29/01/2023), Embase (29/01/2023), and Cochrane CENTRAL (29/01/2023) were searched from inception using free and MeSH term synonyms of “malignant pleural effusion” and “indwelling pleural catheter” plus filters for SRs, randomized controlled trials (RCTs), and cohorts (see supplementary material for the detailed search strategies).
Studies were eligible if they included adult patients with MPE secondary to any metastatic tumor or primary pleural tumors such as mesotheliomas, irrespective of the SCT (chemotherapy, immunotherapy, molecular targeted therapies, or combinations) instituted, and whether or not the lung was trapped. In addition, to be eligible, the intervention for MPE should be ambulatory, and the outcome should have been recorded considering the time of IPC placement in relation to SCT (before, during, or after SCT). Studies with radiotherapy as the only cancer treatment were excluded, as were those in which the results of treated and untreated arms could not be derived separately. Concerning the type of studies, RCTs, quasi-controlled trials, prospective and retrospective cohorts, and case series involving over 20 patients were all eligible. SRs covering the same topic were screened for additional primary studies that had not yet been included. The lists of references of the included studies were likewise scanned for potential further studies that would have been overlooked.
The studies retrieved by the searches were added to an Endnote 21™ (Clarivate Analytics) library and uploaded to the Rayyan® systematic review software (https://www.rayyan.ai/), where a pair of reviewers performed the study selection independently. First, duplicates were removed, after which the titles and abstracts were screened to discard unrelated studies. All remaining reports were read in full, and data were collected.
Data collection, risk of bias assessment, and synthesisFor each study, the eligibility criteria were checked upfront; if these were not met, the study was added to the list of excluded studies, with a comment specifying the unmet criteria.
Data collected from the primary studies were transcribed by one of the reviewers into an Excel® template, which were agreed a priori and cross-checked by the other reviewer. The variables to be collected were related to: (1) study: design, year, country, setting (single center or multicenter), follow-up duration; (2) samplecharacteristics: sample size, cancer type, demographic characteristics (age and gender), and performance status (Karnofsky, Eastern Cooperative Oncology Group); (3) lung and pleural effusion characteristics: expandable/non-expandable lung, pleural loculations, and fluid data (malignant or non-malignant cells, pH, cellular predominance); (4) cancer treatment: use of chemotherapy, immunotherapy, molecular targeted therapy or combinations, and lines of therapy; (5) IPC: indication (symptomatic relief, pleurodesis), drainage schedule (on demand, daily), and catheter implantation setting (inpatient or outpatient); (6) outcomes of interest: efficacy (symptom improvement, need for further procedures, total number of procedures or re-interventions required, hospital stay, patient survival, degree of auto-pleurodesis achieved [different definitions], and time to catheter removal); safety (intra-pleural infection or empyema, and extra-pleural infection including cellulitis, exit site and tunnel tract infections; symptomatic loculations, metastasis of the tract, pain, bleeding, or other complications such as obstruction).
For each outcome, the definition, effect measure, crude, and adjusted values, as well as covariates were collected, and results were expressed by timing groups. After plotting all the results in tables, the researchers met in order to discuss visualization of results and aggregability of studies.
To assess the risk of bias within the included studies, the Cochrane Risk of Bias (RoB) tool for RCTs and Risk of Bias In Non-randomized Studies of Interventions (RoBINS-I) for non-randomized studies were employed. Studies were assessed by a single reviewer and then cross-checked by another.
Meta-analysis was only planned if the studies were sufficiently homogeneous. A random-effects (DerSimonian and Laird) method for meta-analysis was used. Specifically, a meta-analysis on the risk of IPC-related infections from studies which separated data by groups (with and without SCT while IPC was in situ) was performed. Heterogeneity was estimated from an inverse-variance fixed-effect model and presented as I2. The Stata 12.0 (Stata Statistical Software: Release 12. College Station, TX: StataCorp LLC) metaprop and metan commands were used.
ResultsThe search identified 971 records, 10 of which were eventually included (see Fig. 1S in the Supplementary material). Seventy studies that were likely to meet the inclusion criteria based on their title or abstract were excluded following detailed reading. A list of the studies that were excluded with sufficient reasons is detailed in the Supplementary material (Tables 1S and 2S). Most studies were excluded because they did not provide enough data to analyze the outcomes by timing of SCT. The reference lists of three SRs with potentially eligible studies were cross-checked,7,9,10 with no additional studies identified by this or by secondary searches.
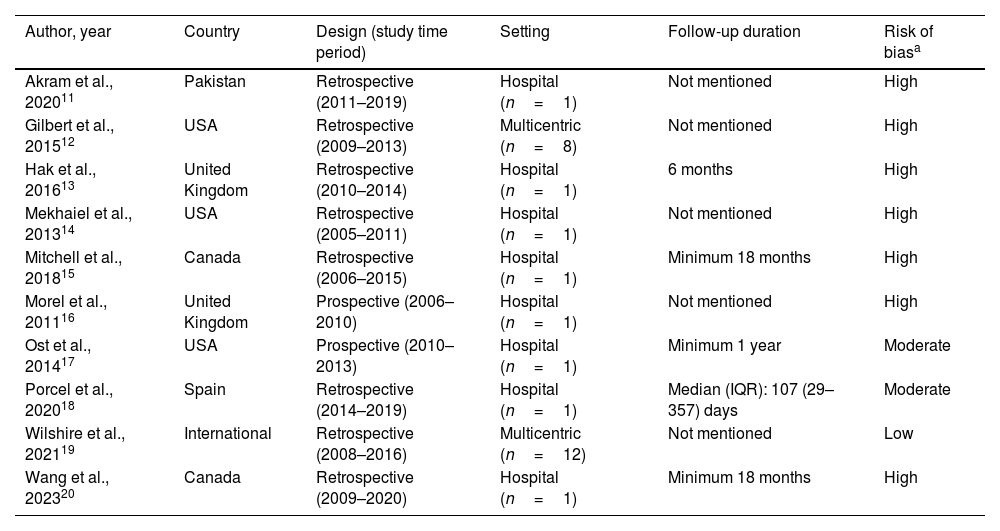
In Table 1, the 10 included studies (n=2907 patients; 3066 IPCs) have been summarized, with their risk of bias being listed.11–20 No RCT was identified, and there were only two studies with a prospective design.16,17 The remaining studies exhibited retrospective designs with variable follow-up times (from a median of 107 days to a minimum of 18 months), and two were multicenter in nature.12,19 Except for the study by Wilshire et al.,19 the remainder displayed moderate to high risk of bias.
Description of the included studies.
| Author, year | Country | Design (study time period) | Setting | Follow-up duration | Risk of biasa |
|---|---|---|---|---|---|
| Akram et al., 202011 | Pakistan | Retrospective (2011–2019) | Hospital (n=1) | Not mentioned | High |
| Gilbert et al., 201512 | USA | Retrospective (2009–2013) | Multicentric (n=8) | Not mentioned | High |
| Hak et al., 201613 | United Kingdom | Retrospective (2010–2014) | Hospital (n=1) | 6 months | High |
| Mekhaiel et al., 201314 | USA | Retrospective (2005–2011) | Hospital (n=1) | Not mentioned | High |
| Mitchell et al., 201815 | Canada | Retrospective (2006–2015) | Hospital (n=1) | Minimum 18 months | High |
| Morel et al., 201116 | United Kingdom | Prospective (2006–2010) | Hospital (n=1) | Not mentioned | High |
| Ost et al., 201417 | USA | Prospective (2010–2013) | Hospital (n=1) | Minimum 1 year | Moderate |
| Porcel et al., 202018 | Spain | Retrospective (2014–2019) | Hospital (n=1) | Median (IQR): 107 (29–357) days | Moderate |
| Wilshire et al., 202119 | International | Retrospective (2008–2016) | Multicentric (n=12) | Not mentioned | Low |
| Wang et al., 202320 | Canada | Retrospective (2009–2020) | Hospital (n=1) | Minimum 18 months | High |
Abbreviation: IQR, interquartile range.
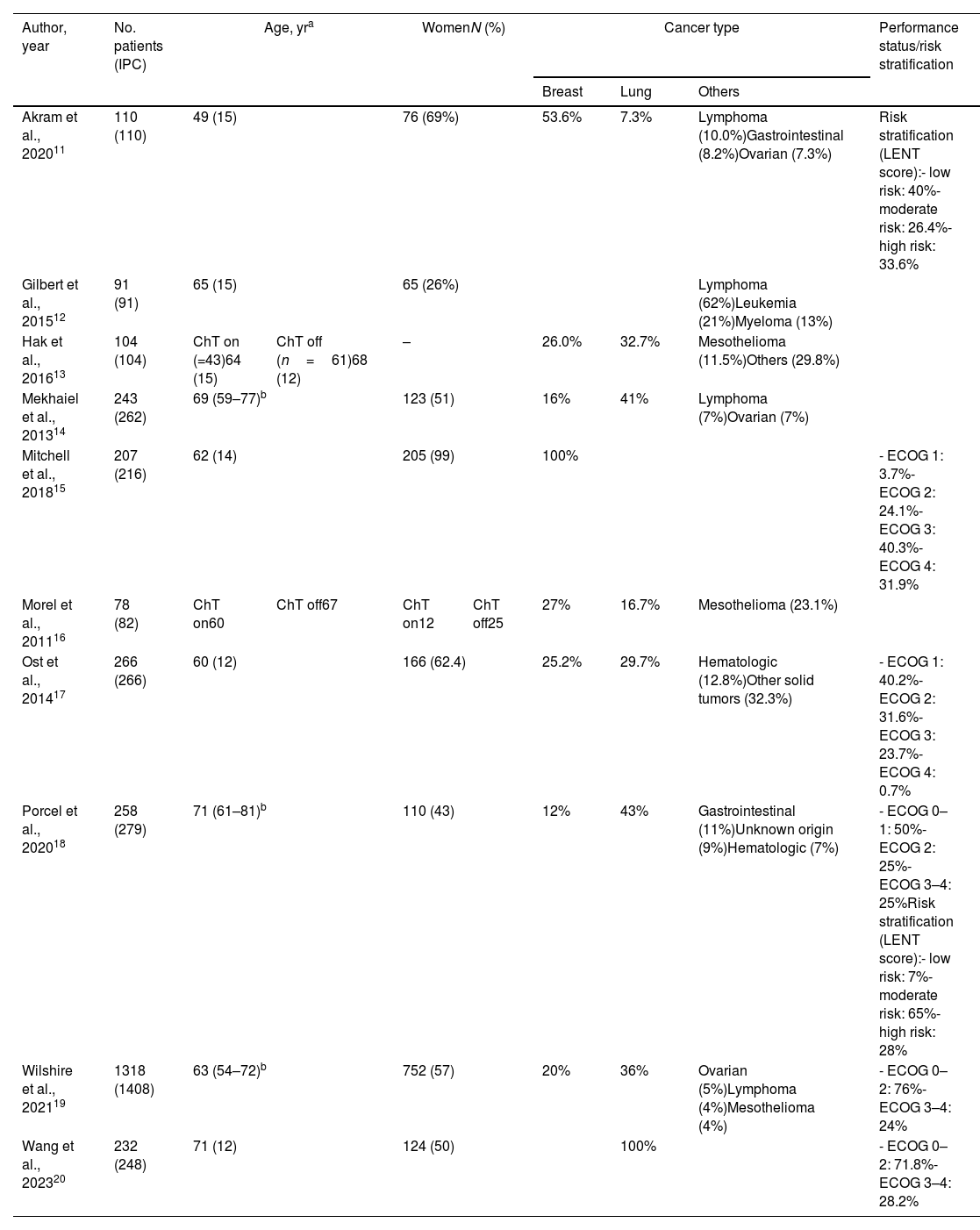
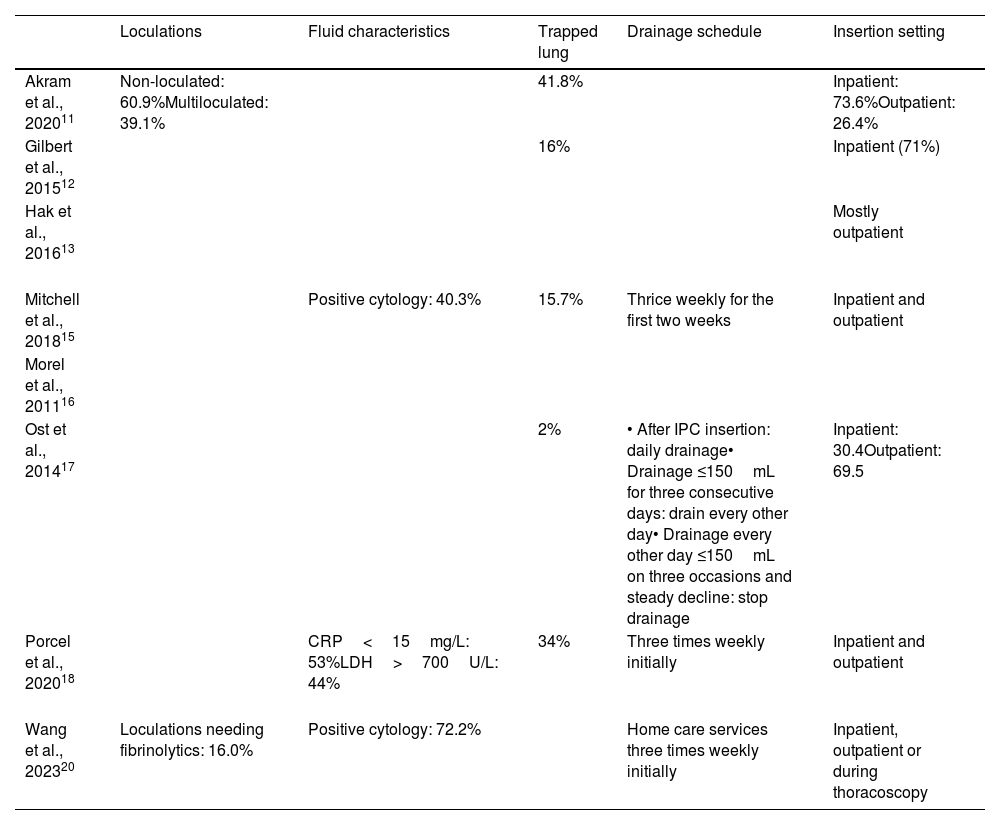
The most common primary tumors were lung (frequency range: 7.3–100%) and breast cancers (12–100%), followed by mesotheliomas, lymphomas or other hematological cancers, ovarian, gastrointestinal, and solid tumors (Table 2). Information about MPE and IPC characteristics was scarce. Loculations were only reported in two studies,11,20 pleural fluid characteristics in three,15,18,20 while the percentage of trapped lung ranged from 2% to 42%,11,12,15,18 with IPC insertion being mainly ambulatory (Table 3). Regarding SCT, different treatments (chemotherapy, radiotherapy, immunotherapy, molecular targeted therapies) with different timings in relation to IPC insertion (before IPC, while IPC was in place, or after IPC removal) were reported. All studies comprised at least one group of patients receiving SCT, but the most common comparison groups were SCT vs no SCT whilst IPC was in situ (Table 4).
Included studies: sample characteristics.
| Author, year | No. patients (IPC) | Age, yra | WomenN (%) | Cancer type | Performance status/risk stratification | ||||
|---|---|---|---|---|---|---|---|---|---|
| Breast | Lung | Others | |||||||
| Akram et al., 202011 | 110 (110) | 49 (15) | 76 (69%) | 53.6% | 7.3% | Lymphoma (10.0%)Gastrointestinal (8.2%)Ovarian (7.3%) | Risk stratification (LENT score):- low risk: 40%- moderate risk: 26.4%- high risk: 33.6% | ||
| Gilbert et al., 201512 | 91 (91) | 65 (15) | 65 (26%) | Lymphoma (62%)Leukemia (21%)Myeloma (13%) | |||||
| Hak et al., 201613 | 104 (104) | ChT on (=43)64 (15) | ChT off (n=61)68 (12) | – | 26.0% | 32.7% | Mesothelioma (11.5%)Others (29.8%) | ||
| Mekhaiel et al., 201314 | 243 (262) | 69 (59–77)b | 123 (51) | 16% | 41% | Lymphoma (7%)Ovarian (7%) | |||
| Mitchell et al., 201815 | 207 (216) | 62 (14) | 205 (99) | 100% | - ECOG 1: 3.7%- ECOG 2: 24.1%- ECOG 3: 40.3%- ECOG 4: 31.9% | ||||
| Morel et al., 201116 | 78 (82) | ChT on60 | ChT off67 | ChT on12 | ChT off25 | 27% | 16.7% | Mesothelioma (23.1%) | |
| Ost et al., 201417 | 266 (266) | 60 (12) | 166 (62.4) | 25.2% | 29.7% | Hematologic (12.8%)Other solid tumors (32.3%) | - ECOG 1: 40.2%- ECOG 2: 31.6%- ECOG 3: 23.7%- ECOG 4: 0.7% | ||
| Porcel et al., 202018 | 258 (279) | 71 (61–81)b | 110 (43) | 12% | 43% | Gastrointestinal (11%)Unknown origin (9%)Hematologic (7%) | - ECOG 0–1: 50%- ECOG 2: 25%- ECOG 3–4: 25%Risk stratification (LENT score):- low risk: 7%- moderate risk: 65%- high risk: 28% | ||
| Wilshire et al., 202119 | 1318 (1408) | 63 (54–72)b | 752 (57) | 20% | 36% | Ovarian (5%)Lymphoma (4%)Mesothelioma (4%) | - ECOG 0–2: 76%- ECOG 3–4: 24% | ||
| Wang et al., 202320 | 232 (248) | 71 (12) | 124 (50) | 100% | - ECOG 0–2: 71.8%- ECOG 3–4: 28.2% | ||||
Abbreviations: ChT, chemotherapy; ECOG, Eastern Cooperative Oncology Group; IPC, indwelling pleural catheter; LENT (L=LDH in pleural fluid; E=ECOG performance status; NLR=neutrophil to lymphocyte ratio; T=tumor type). In papers without information on the number of IPCs inserted at least the same number of procedures as patients was assumed.
Characteristics of pleural effusions and indwelling pleural catheters in the included studies, where available.
| Loculations | Fluid characteristics | Trapped lung | Drainage schedule | Insertion setting | |
|---|---|---|---|---|---|
| Akram et al., 202011 | Non-loculated: 60.9%Multiloculated: 39.1% | 41.8% | Inpatient: 73.6%Outpatient: 26.4% | ||
| Gilbert et al., 201512 | 16% | Inpatient (71%) | |||
| Hak et al., 201613 | Mostly outpatient | ||||
| Mitchell et al., 201815 | Positive cytology: 40.3% | 15.7% | Thrice weekly for the first two weeks | Inpatient and outpatient | |
| Morel et al., 201116 | |||||
| Ost et al., 201417 | 2% | • After IPC insertion: daily drainage• Drainage ≤150mL for three consecutive days: drain every other day• Drainage every other day ≤150mL on three occasions and steady decline: stop drainage | Inpatient: 30.4Outpatient: 69.5 | ||
| Porcel et al., 202018 | CRP<15mg/L: 53%LDH>700U/L: 44% | 34% | Three times weekly initially | Inpatient and outpatient | |
| Wang et al., 202320 | Loculations needing fibrinolytics: 16.0% | Positive cytology: 72.2% | Home care services three times weekly initially | Inpatient, outpatient or during thoracoscopy |
Abbreviations: CRP, C-reactive protein; LDH, lactate dehydrogenase.
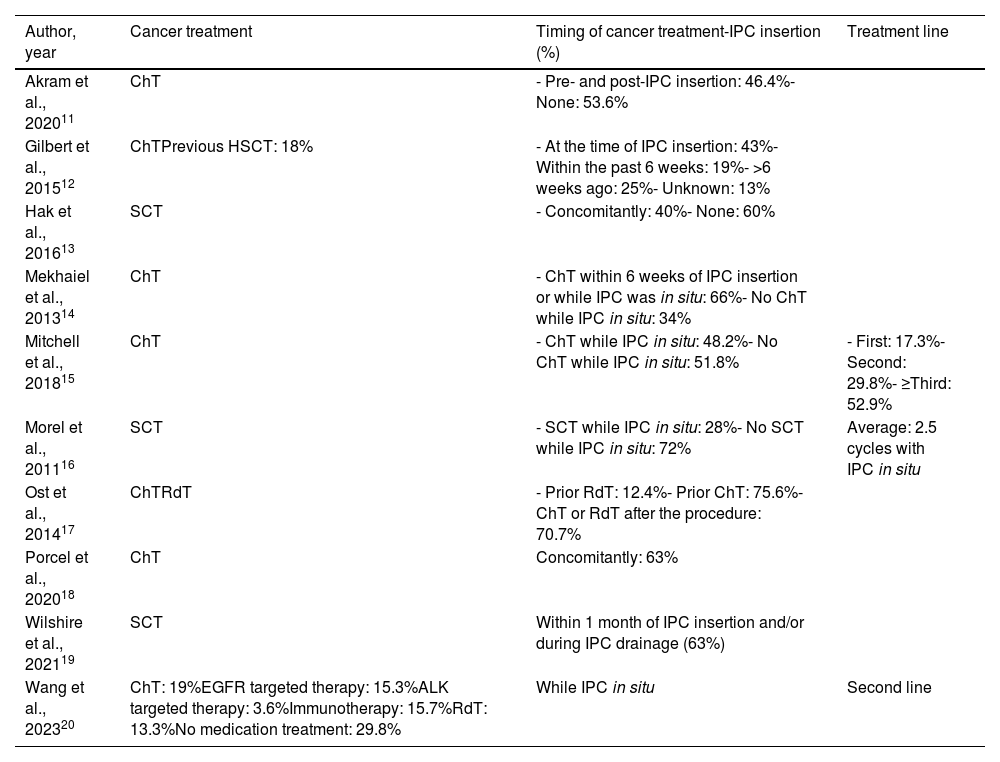
Characteristics of cancer treatments in the included studies.
| Author, year | Cancer treatment | Timing of cancer treatment-IPC insertion (%) | Treatment line |
|---|---|---|---|
| Akram et al., 202011 | ChT | - Pre- and post-IPC insertion: 46.4%- None: 53.6% | |
| Gilbert et al., 201512 | ChTPrevious HSCT: 18% | - At the time of IPC insertion: 43%- Within the past 6 weeks: 19%- >6 weeks ago: 25%- Unknown: 13% | |
| Hak et al., 201613 | SCT | - Concomitantly: 40%- None: 60% | |
| Mekhaiel et al., 201314 | ChT | - ChT within 6 weeks of IPC insertion or while IPC was in situ: 66%- No ChT while IPC in situ: 34% | |
| Mitchell et al., 201815 | ChT | - ChT while IPC in situ: 48.2%- No ChT while IPC in situ: 51.8% | - First: 17.3%- Second: 29.8%- ≥Third: 52.9% |
| Morel et al., 201116 | SCT | - SCT while IPC in situ: 28%- No SCT while IPC in situ: 72% | Average: 2.5 cycles with IPC in situ |
| Ost et al., 201417 | ChTRdT | - Prior RdT: 12.4%- Prior ChT: 75.6%- ChT or RdT after the procedure: 70.7% | |
| Porcel et al., 202018 | ChT | Concomitantly: 63% | |
| Wilshire et al., 202119 | SCT | Within 1 month of IPC insertion and/or during IPC drainage (63%) | |
| Wang et al., 202320 | ChT: 19%EGFR targeted therapy: 15.3%ALK targeted therapy: 3.6%Immunotherapy: 15.7%RdT: 13.3%No medication treatment: 29.8% | While IPC in situ | Second line |
Abbreviations: ALK, anaplastic lymphoma kinase; ChT, chemotherapy; EGFR, epidermal grow factor receptor; HSCT, hematopoietic stem cell transplantation; IPC, indwelling pleural catheter; RdT, radiotherapy; SCT, systemic cancer therapy.
Efficacy and safety outcomes were evaluated, even though they were not always documented in all studies.
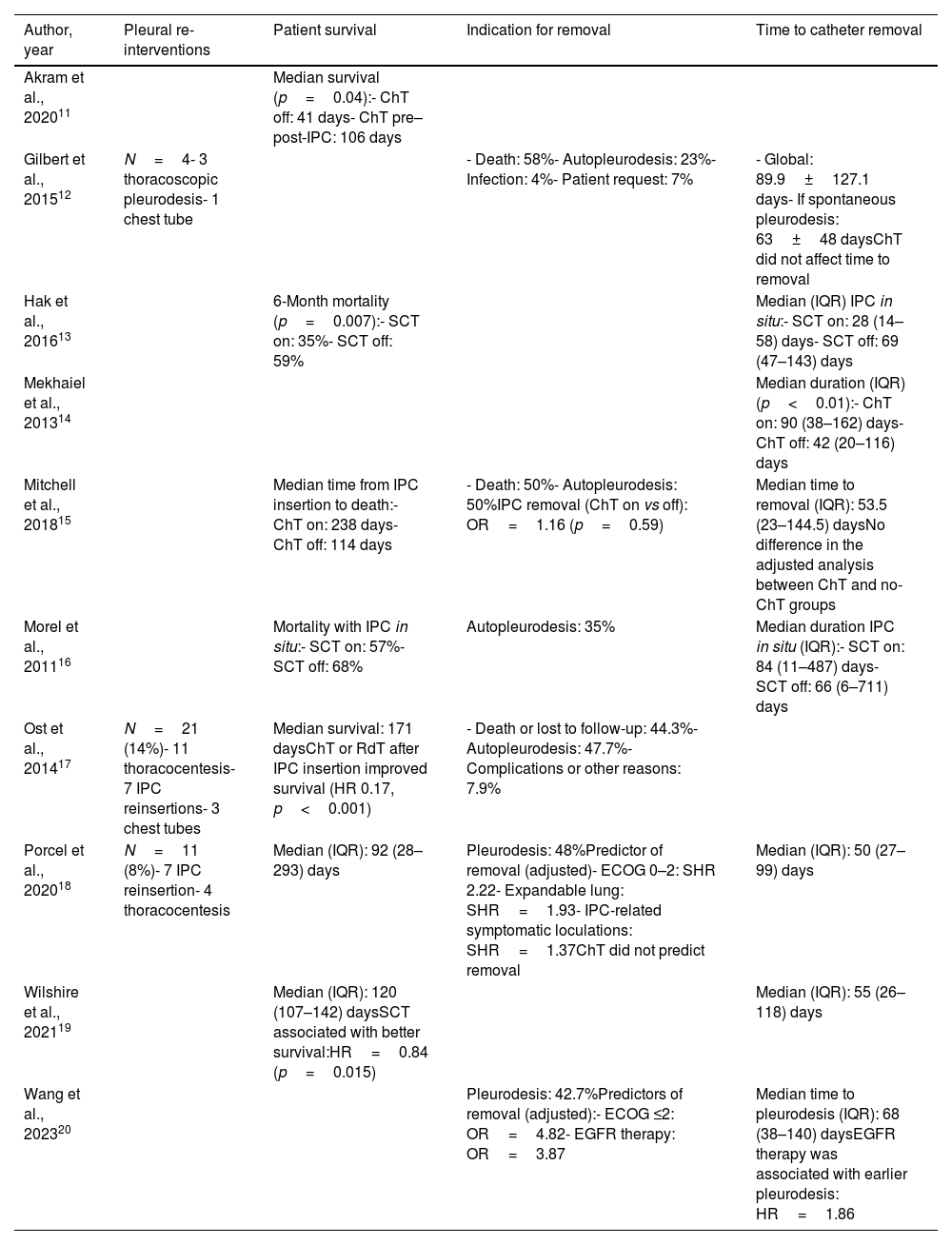
Given there were no comparative studies included, it was not possible to assess the efficacy related to symptom improvement or decreased hospitalization days in IPC carriers undergoing SCT. Of the included studies, only three reported re-intervention procedures,12,17,18 seven patient survival,11,13,15–19 six indication for catheter removal,12,15–18,20 and eight time to catheter removal12–16,18–20 (Table 5).
Outcomes: indwelling pleural catheter efficacy.
| Author, year | Pleural re-interventions | Patient survival | Indication for removal | Time to catheter removal |
|---|---|---|---|---|
| Akram et al., 202011 | Median survival (p=0.04):- ChT off: 41 days- ChT pre–post-IPC: 106 days | |||
| Gilbert et al., 201512 | N=4- 3 thoracoscopic pleurodesis- 1 chest tube | - Death: 58%- Autopleurodesis: 23%- Infection: 4%- Patient request: 7% | - Global: 89.9±127.1 days- If spontaneous pleurodesis: 63±48 daysChT did not affect time to removal | |
| Hak et al., 201613 | 6-Month mortality (p=0.007):- SCT on: 35%- SCT off: 59% | Median (IQR) IPC in situ:- SCT on: 28 (14–58) days- SCT off: 69 (47–143) days | ||
| Mekhaiel et al., 201314 | Median duration (IQR) (p<0.01):- ChT on: 90 (38–162) days- ChT off: 42 (20–116) days | |||
| Mitchell et al., 201815 | Median time from IPC insertion to death:- ChT on: 238 days- ChT off: 114 days | - Death: 50%- Autopleurodesis: 50%IPC removal (ChT on vs off): OR=1.16 (p=0.59) | Median time to removal (IQR): 53.5 (23–144.5) daysNo difference in the adjusted analysis between ChT and no-ChT groups | |
| Morel et al., 201116 | Mortality with IPC in situ:- SCT on: 57%- SCT off: 68% | Autopleurodesis: 35% | Median duration IPC in situ (IQR):- SCT on: 84 (11–487) days- SCT off: 66 (6–711) days | |
| Ost et al., 201417 | N=21 (14%)- 11 thoracocentesis- 7 IPC reinsertions- 3 chest tubes | Median survival: 171 daysChT or RdT after IPC insertion improved survival (HR 0.17, p<0.001) | - Death or lost to follow-up: 44.3%- Autopleurodesis: 47.7%- Complications or other reasons: 7.9% | |
| Porcel et al., 202018 | N=11 (8%)- 7 IPC reinsertion- 4 thoracocentesis | Median (IQR): 92 (28–293) days | Pleurodesis: 48%Predictor of removal (adjusted)- ECOG 0–2: SHR 2.22- Expandable lung: SHR=1.93- IPC-related symptomatic loculations: SHR=1.37ChT did not predict removal | Median (IQR): 50 (27–99) days |
| Wilshire et al., 202119 | Median (IQR): 120 (107–142) daysSCT associated with better survival:HR=0.84 (p=0.015) | Median (IQR): 55 (26–118) days | ||
| Wang et al., 202320 | Pleurodesis: 42.7%Predictors of removal (adjusted):- ECOG ≤2: OR=4.82- EGFR therapy: OR=3.87 | Median time to pleurodesis (IQR): 68 (38–140) daysEGFR therapy was associated with earlier pleurodesis: HR=1.86 |
Abbreviations: ChT, chemotherapy; ECOG, Eastern Cooperative Oncology Group; EGFR, epidermal grow factor receptor; HR, hazard ratio; IQR, interquartile range; IPC, indwelling pleural catheter; RdT, radiotherapy; SCT, systemic cancer therapy; SHR, sub-hazard ratio.
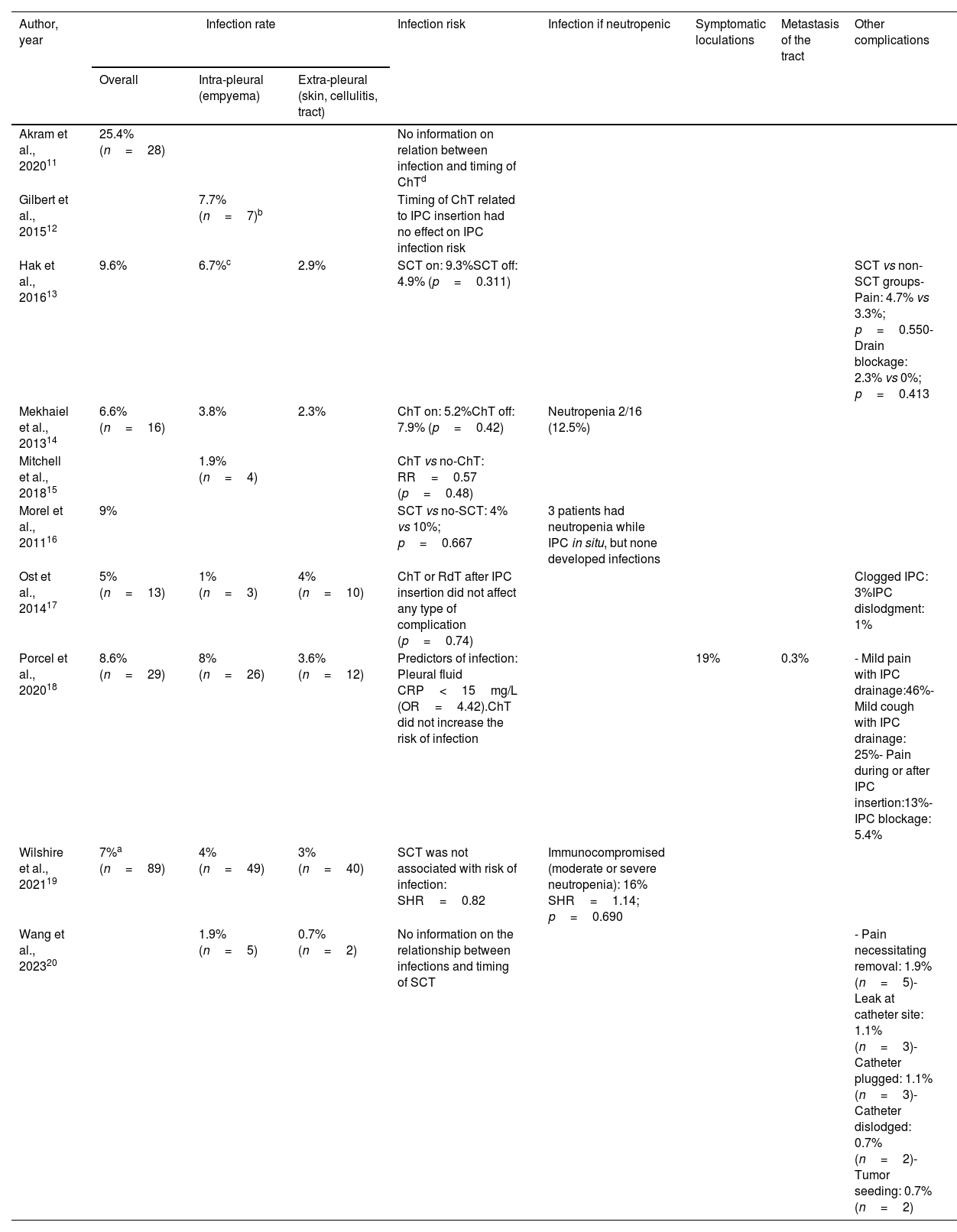
Safety data were collected by assessing the following information: intra- or extra-pleural infections (all the included studies), symptomatic loculations,18 metastasis of the tract,18 and other complications13,17,18,20 (Table 6).
Outcomes: indwelling pleural catheter safety.
| Author, year | Infection rate | Infection risk | Infection if neutropenic | Symptomatic loculations | Metastasis of the tract | Other complications | ||
|---|---|---|---|---|---|---|---|---|
| Overall | Intra-pleural (empyema) | Extra-pleural (skin, cellulitis, tract) | ||||||
| Akram et al., 202011 | 25.4% (n=28) | No information on relation between infection and timing of ChTd | ||||||
| Gilbert et al., 201512 | 7.7% (n=7)b | Timing of ChT related to IPC insertion had no effect on IPC infection risk | ||||||
| Hak et al., 201613 | 9.6% | 6.7%c | 2.9% | SCT on: 9.3%SCT off: 4.9% (p=0.311) | SCT vs non-SCT groups- Pain: 4.7% vs 3.3%; p=0.550- Drain blockage: 2.3% vs 0%; p=0.413 | |||
| Mekhaiel et al., 201314 | 6.6% (n=16) | 3.8% | 2.3% | ChT on: 5.2%ChT off: 7.9% (p=0.42) | Neutropenia 2/16 (12.5%) | |||
| Mitchell et al., 201815 | 1.9% (n=4) | ChT vs no-ChT: RR=0.57 (p=0.48) | ||||||
| Morel et al., 201116 | 9% | SCT vs no-SCT: 4% vs 10%; p=0.667 | 3 patients had neutropenia while IPC in situ, but none developed infections | |||||
| Ost et al., 201417 | 5% (n=13) | 1% (n=3) | 4% (n=10) | ChT or RdT after IPC insertion did not affect any type of complication (p=0.74) | Clogged IPC: 3%IPC dislodgment: 1% | |||
| Porcel et al., 202018 | 8.6% (n=29) | 8% (n=26) | 3.6% (n=12) | Predictors of infection: Pleural fluid CRP<15mg/L (OR=4.42).ChT did not increase the risk of infection | 19% | 0.3% | - Mild pain with IPC drainage:46%- Mild cough with IPC drainage: 25%- Pain during or after IPC insertion:13%- IPC blockage: 5.4% | |
| Wilshire et al., 202119 | 7%a (n=89) | 4% (n=49) | 3% (n=40) | SCT was not associated with risk of infection: SHR=0.82 | Immunocompromised (moderate or severe neutropenia): 16% SHR=1.14; p=0.690 | |||
| Wang et al., 202320 | 1.9% (n=5) | 0.7% (n=2) | No information on the relationship between infections and timing of SCT | - Pain necessitating removal: 1.9% (n=5)- Leak at catheter site: 1.1% (n=3)- Catheter plugged: 1.1% (n=3)- Catheter dislodged: 0.7% (n=2)- Tumor seeding: 0.7% (n=2) | ||||
Abbreviations: ChT, chemotherapy; CRP, C-reactive protein; IPC, indwelling pleural catheter; OR, odds ratio; RdT, radiotherapy; SCT, systemic cancer therapy; SHR, sub-hazard ratio.
Survival was expressed as median survival time, 6-month mortality, and mortality with IPC in situ. All studies demonstrated a more prolonged survival in those patients in whom the IPC was inserted close to SCT or during SCT.11,13,15–19
Akram et al. reported that patients who received chemotherapy before and after IPC insertion exhibited longer median survival in comparison with those who did not receive any oncological treatment (106 vs 41 days; p=0.004).11 Consequently, the decision regarding institution of the oncological treatment is an essential factor contributing to survival. According to Hak et al. the 6-month mortality was lower in patients with IPC and concomitant SCT than in those without SCT (35% vs 59%; p=0.007).13 The results of Morel et al. pointed toward the same direction, with lower mortality rates in patients undergoing SCT upon IPC insertion compared with those who did not receive SCT (57% vs 68%), although there was no mention of whether this difference was statistically significant.16 The study by Ost et al. demonstrated an improvement in quality-adjusted survival in patients starting chemo- or radiotherapy immediately after IPC insertion.17 Mitchell et al. showed that chemotherapy in IPC carriers increased survival time (238 vs 114 days),15 defined as the median time from IPC insertion to death. Finally, Whilshire et al. observed that SCT within a month of IPC insertion or during IPC use was associated with a 16% increase in survival (hazard ratio [HR]=0.84; p=0.015).19
Conducting a meta-analysis was not considered, given the variability of the outcomes and definition of the interventions.
Indication to remove IPC and duration of IPC insertionDeath and auto-pleurodesis were the most common causes of IPC removal.12,15–18,20 The rate of spontaneous pleurodesis was high, ranging between 23% and 50% (Table 5). Although using concomitant chemotherapy when the IPC was in situ did not impact probability of removal in two studies,15,18 Wang et al. observed greater rates of spontaneous pleurodesis in patients with lung cancer who were subjected to epidermal growth factor receptor (EGFR) therapy while the IPC was in situ (adjusted OR=3.87)20 (Table 5).
On the other hand, information on the duration of the IPC in situ was not fully consistent.12–16,18–20 Although the variability of the studies does not allow drawing solid conclusions, the results suggested longer catheter duration in patients undergoing SCT, except for the Hak et al. study.13 However, the differences were only statistically significant in the Mekhaiel et al. study14 and could be related to longer survival with chemotherapy. Concerning the remaining studies, multivariate analyses either did not reveal differences between patients with or without SCT upon IPC placement15 or the statistical significance of the comparison was not recorded.13,16,18,19
Performing a meta-analysis was not taken into consideration, owing to the difficulty of pooling median times and interquartile ranges, which were the most reported effect measures.
Re-interventionsThree studies presented data about additional pleural procedures being required. Overall, the frequency of pleural re-interventions was low, but the results were mainly descriptive, without any comparison between patients with or without chemotherapy while the IPC was insitu.12,17,18 Nevertheless, in the study by Gilbert et al.,12 where all 91 patients included underwent chemotherapy, no additional thoracocentesis was reported, and there was only one placement of a chest tube (1.1%), and 3 thoracoscopic pleurodesis procedures (3.3%) (Table 5).
IPC-related infectionsAll the included studies presented data on IPC-related infections, whether intrapleural (empyema) or extrapleural (cellulitis, exit site infection or line tract infection). Nevertheless, the direct effect of SCT and the precise time of infection during treatment were not properly evaluated by all the authors.
The overall infection prevalence was highly variable, which can probably be accounted for by methodological differences among studies. Overall, IPC-related infections ranged from 1.9%15 to as high as 25.4% in one publication,11 but in the latter the authors did not specify the infection sites. By infection location, the presence of empyema as a complication of IPC ranged from 1%17 to 8%,12 while infections pertaining to the extra-pleural space (cellulitis) exhibited less variation, from 0.7%14 to 4%.17 The pooled estimated rate of IPC-related infections was 2.85% (95% confidence interval: 2.24–3.45; I2 73.39%) (Fig. 2S).
Two of the 10 included studies did not present data on the possible relationship between infection occurrence and SCT timing.11,20 The Akram et al. study reported a significant reduction (p=0.03) in the infection rate of patients receiving domiciliary care education, but the infection frequency was not analyzed in relation with the chemotherapy timing.11 Wang et al. reported a prevalence of intra-pleural infection of 1.9%, also without information on its possible relationship with SCT timing.20 The association between complications and SCT timing was analyzed globally, clearly demonstrating that SCT with concurrent or sequential radiotherapy following IPC insertion did not at all increase the overall risk of complications. The association between infection and SCT timing in relation to IPC insertion was analyzed in eight of the 10 studies.12–19 None of them found a significant increase in infection risk associated with the SCT timing, despite the presence of neutropenia12–19 (Table 6). A meta-analysis showed that the pooled relative risk (RR) of IPC-related infections while on SCT compared to off-therapy was 0.98 (95% CI: 0.93–1.03; I2 0.0%) (Fig. 3S).
Other outcomes comprised IPC-related pain and drain blockage, without any effect of SCT timing,13 in addition to symptomatic loculations, metastasis of the tract, leaks, or tumor seeding which were not analyzed with respect to SCT.18,20
DiscussionIPC placement, as an early measure in MPE management, is an adequate option to relief symptoms, maintain quality of life and reduce hospital stays, while potentially reducing the costs related to the latter.21 However, definitive MPE management is not without complications and the optimal timing of IPC insertion with respect to SCT is still controversial. Some clinicians advocate delaying MPE control, while expecting the response to SCT, whereas others argue that MPE recurrences are the rule, thus resulting in deterioration of the patient's condition and reducing the possibility of further definitive interventions owing to lung entrapment.22 This systematic review compared the safety and effectiveness of IPCs in relation to the timing of antineoplastic treatment, mainly consisting of chemotherapy (before IPC insertion, while IPC was in situ, and after IPC removal).
Results show that IPC carriers undergoing SCT have improved survival, irrespective of the timing of treatment (pre- and post-IPC insertion, concomitant with IPC, after IPC, within 1 month of insertion, or during IPC drainage), although these results could also be due to the better functional status of patients with IPC who are able to receive SCT. Moreover, the rate of auto-pleurodesis reported with targeted therapy to EGFR mutations was very high,20 suggesting that highly effective therapies and overall good disease control increase the probability of spontaneous pleurodesis and early removal of the IPC. In turn, chemotherapy was not a predictor of pleurodesis in the studies included in the current review.15,18
Infection is one of the most common complications of IPC, and the prevalence of infections and their relationship with SCT are of paramount importance. Meta-analytical data from this systematic review suggest that SCT timing exerts no effect on the risk of IPC-related complications, including infections, even in immunocompromised patients with treatment-induced neutropenia.
These results are in concordance with the scientific literature. In a longitudinal study involving newly diagnosed MPE due to lung cancer, Chiang et al. demonstrated that early MPE control measures were beneficial at reducing the frequency of further interventions, regardless of the anti-cancer therapies used, including molecular targeted therapy.21 Other studies have also supported the use of definitive pleural treatments (IPC or pleurodesis) in lung cancer patients with oncogenic mutations, without deferring until the eventual response to targeted therapies become apparent.23 In a retrospective analysis of MPEs from different tumor types, Holling et al. observed that SCT was not independently associated with MPE resolution, defined by radiologic resolution with removal of drain or catheter and cessation of interventions.24 Based on these results, the authors stated that all patients with MPE should be offered early definitive pleural interventions, regardless of the oncologic treatment plan,1,25,26 as an integral part of the early palliative care program.
This systematic review has a few limitations. In general, the quality of the included studies was low to moderate, rendering it necessary to downsize the interpretation of results. First, none of the included studies was a RCT, but rather they were mainly retrospective studies, with only two having a prospective design. Second, most of the studies were conducted at a single center. Therefore, operator-dependent, or other center-related variables may have caused potential selection biases. Other limitations were related to the studies’ statistical power that was insufficient to detect differences, along with inadequate handling of potential confounders. Finally, it was not possible to perform a meta-analysis of some outcomes of clinical interest, such as the time to IPC removal according to the simultaneous administration or nor of SCT.
In view of these results, it is crucial to encourage the scientific community to design a proper and pragmatic RCT that evaluates the true influence of diverse SCT on MPE resolution and, therefore, the role of early IPC placement in this setting. International collaboration with prospective standardized databases or registries would allow for more sounded study designs.
In summary, based on observational evidence, it seems that IPC insertion in patients with MPE should not be delayed pending the beneficial effect of SCT, if any, on pleural fluid clearance. In particular, there should be no concern among clinicians about a potential increased risk of IPC infections in patients undergoing SCT compared to those not receiving SCT, as this has not been demonstrated.
Registration and protocolThe review protocol was registered in PROSPERO, prior to the search strategy, under number CRD42022322026.
Availability of data, code, and other materialsThe Endnote® libraries and data extraction forms are available upon reasonable request.
FundingThe study was funded by an unrestricted grant from SH Medical Group.
Conflict of interestsAll the authors received consultancy fees from SH medical. In addition, JMP received consultancy fees from Becton Dickinson; RC received honoraria for lectures and participation to advisory boards (Olympus, SH medical, and Spanish Society of Pneumology and Thoracic Surgery).
DBB, JPP, RMT, JFA, ECB, BR, and MBR have no other conflicts of interest.
We acknowledge the help of both María Luisa Maquedano and María García-Puente (Alter Biblio) who performed the search strategies, and of both María Jesús García de Yébenes and Loreto Carmona (Inmusc), who performed the study selection, data collection, risk of bias assessment, and data synthesis.