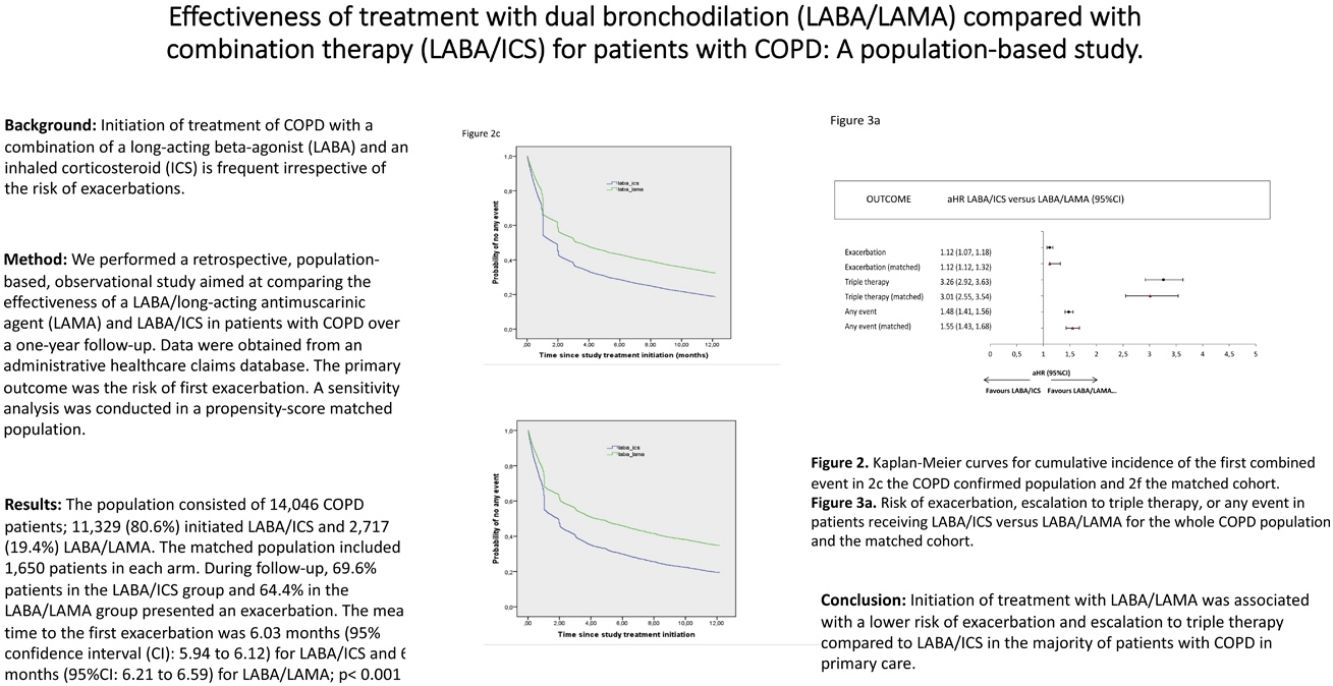
Initiation of treatment of COPD with a combination of a long-acting beta-agonist (LABA) and an inhaled corticosteroid (ICS) is frequent irrespective of the risk of exacerbations.
MethodWe performed a retrospective, population-based, observational study aimed at comparing the effectiveness of a LABA/long-acting antimuscarinic agent (LAMA) and LABA/ICS in patients with COPD over a one-year follow-up. Data were obtained from an administrative healthcare claims database. The primary outcome was the risk of first exacerbation. A sensitivity analysis was conducted in a propensity-score matched population.
ResultsThe population consisted of 14,046 COPD patients; 11,329 (80.6%) initiated LABA/ICS and 2717 (19.4%) LABA/LAMA. The matched population included 1650 patients in each arm. During follow-up, 69.6% patients in the LABA/ICS group and 64.4% in the LABA/LAMA group presented an exacerbation. The mean time to the first exacerbation was 6.03 months (95% confidence interval (CI): 5.94–6.12) for LABA/ICS and 6.4 months (95%CI: 6.21–6.59) for LABA/LAMA; p<0.001. The time to scalation to triple therapy was also significantly prolonged in LABA/LAMA. Similar results were obtained in the matched population. LABA/LAMA was associated with a significantly lower risk of exacerbations and escalation to triple therapy compared to LABA/ICS, except in patients with frequent exacerbations and high blood eosinophils in which no differences were observed in the time to first exacerbation.
ConclusionInitiation of treatment with LABA/LAMA was associated with a lower risk of exacerbation and escalation to triple therapy compared to LABA/ICS in the majority of patients with COPD in primary care.
Long-acting bronchodilators are the basis of treatment of chronic obstructive pulmonary disease (COPD).1 They improve lung function, reduce symptoms, and increase exercise capacity1,2; however, some patients may present with recurrent episodes of exacerbation and require an inhaled corticosteroid (ICS).3 Current guidelines and consensus documents recommend the use of a combination of a long-acting beta-agonist (LABA) and an ICS as initial therapy in COPD patients with exacerbations and an eosinophilic profile of inflammation demonstrated by a concentration of blood eosinophils higher than 300cells/mL.4,5
This recommendation is based on results from different randomized clinical trials, although some have provided conflicting results. One trial demonstrated that a single long-acting antimuscarinic agent (LAMA) may be as effective as a combination of LABA/ICS for the prevention of exacerbations.6 Another trial showed that a combination of LABA/LAMA was superior to LABA/ICS in the prevention of exacerbations of COPD irrespective of blood eosinophil concentrations.7,8 In contrast, two more recent large trials have shown that the combination of LABA/ICS may be more effective than LABA/LAMA in the prevention of exacerbations.9,10 It is important to recognize that the patients included in these trials have different characteristics in terms of severity, frequency of previous exacerbations and previous treatments; in addition, most of these studies were conducted in tertiary hospitals and the populations included may not be representative of patients in primary care.
A recent retrospective study conducted in a US claims database reported that treatment with the tiotropium/olodaterol LAMA/LABA combination was associated with a lower risk of COPD exacerbations, pneumonia, and escalation to triple therapy compared with LABA/ICS, and this risk was reduced irrespective of baseline eosinophils and exacerbation history.11 These results suggest that patients attended in primary care, who are generally milder and infrequent exacerbators12,13 may benefit more from dual bronchodilation than LABA/ICS, in contrast to patients recruited in tertiary hospitals who are usually more severe and frequent exacerbators and may respond better to ICS.
This observation is relevant to reducing inappropriate long-term use of ICS in primary care, which may be associated with a series of side effects.14 Therefore, this non-interventional database study aimed to investigate the risk of COPD exacerbations, hospitalization and escalation to triple therapy in patients initiating LABA/LAMA or LABA/ICS in primary care.
MethodStudy designThis was a retrospective, population-based, observational study aimed at comparing the effectiveness of maintenance treatment with LABA/LAMA and LABA/ICS in patients with COPD in Catalonia (Spain). Data were obtained from a large administrative healthcare claims database and outcomes were measured during the year after initiation of either LABA/LAMA or LABA/ICS fixed combinations for maintenance therapy of COPD. The study was approved by the Research and Ethics Committee of the IDIAP Jordi Gol Institute of Research In Primary Care (Barcelona, Spain). Since anonymised data were collected retrospectively, no informed consent was considered necessary.
Data sourceThe data was obtained from SIDIAP database (Information System for the Development of Research in Primary Care) which contains anonymized computerized primary care medical records from 5.8 million people in Catalonia, representing more than 80% of the total population.15 The SIDIAP database provides information from medical records (demographic data, past medical history, visits to the general practitioner (GP), clinical variables, prescriptions, immunizations, and referrals), laboratory results and medication dispensed. It is also linked to other databases in Catalonia, such as the hospital discharge summaries database and the mortality registry. The SIDIAP database has been validated and extensively used for other studies on COPD.16,17
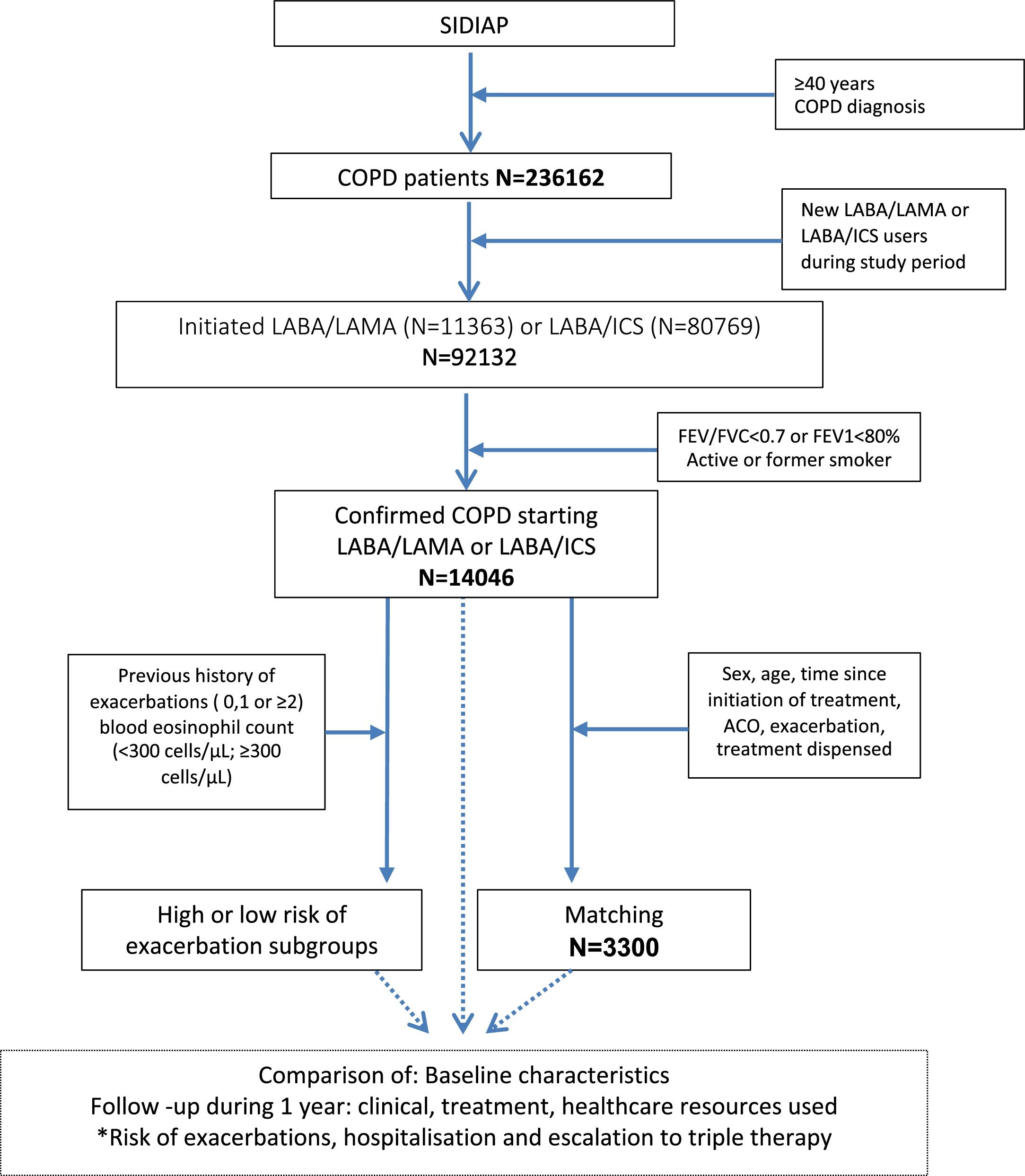
Patient selectionThe study inclusion criteria were: (1) adults of both sexes older than 40 years; (2) at least one diagnosis of COPD (International Classification of Disease – 10th Edition [ICD-10] codes J41, J42, J43 and J44); (3) spirometry with forced expiratory volume in 1 second (FEV1)/forced vital capacity (FVC)<0.7; (4) in smokers or former smokers; (5) at least one dispensation for LABA/LAMA (fixed form) or a LABA/ICS (fixed form) between 1 January 2013 and 31 December 2019, with the first prescription defined as the index date; (6) a minimum follow-up period of 3 months after initiation of treatment; and (7) at least 12 month of available medical record data prior to the index date (“pre-index”).
The exclusion criteria were: (1) patients with any evidence of LABA/LAMA or LABA/ICS or LABA/LAMA/ICS prescriptions in free or fixed form during the pre-index period; (2) diagnosis of asthma in the year prior to the index date; (3) diagnosis of lung cancer, interstitial lung disease, or lung transplant identified at any time prior to the index date; and (4) a COPD exacerbation 30 days before the index date.
Study subpopulationsPatients were classified according to the Global Initiative for Chronic Obstructive lung Disease (GOLD) spirometric categories based on FEV1(% predicted) as GOLD I–IV.4 Patients were also classified according to clinical phenotypes.18 Those with two or more exacerbations during the year before the index date were classified as having a frequent exacerbator phenotype, while patients with only one exacerbation were classified as infrequent exacerbators and the remaining COPD patients were considered non-exacerbators. Patients with a concomitant diagnosis of asthma were included in the asthma COPD overlap (ACO) phenotype.19,20
All analyses were conducted for the total population as well as in subgroups of patients at high or low risk of exacerbations based on (1) previous history of exacerbations in the year preceding cohort entry (low exacerbation risk: 0 hospitalisations and 0–1 outpatient exacerbations; high exacerbation risk: ≥1 hospitalization or ≥2 outpatient exacerbations); and (2) blood eosinophil count (baseline eosinophils<300cells/μL or ≥300cells/μL) as identified based on the laboratory result value that was closest but prior to the index date (within 6 months). Finally, according to both variables, patients were classified into four subgroups of high/low risk of exacerbation with high/low blood eosinophil counts.
Exposure measuresExposure measures were based on pharmacy dispensation of LABA/LAMA and LABA/ICS over up to 1 year of follow-up. Exposure was based on the current use of LABA/LAMA or LABA/ICS as defined by the dispensation date plus the day's supply recorded at the time of dispensation.
A gap of 15 days was allowed between dispensations to allow for delays in obtaining medication refills and for continued use beyond the days supplied on which medication had been missed due to imperfect adherence.
Patients were followed from the index date until the earliest of the following: first occurrence of a COPD exacerbation, hospitalization for community-acquired pneumonia, escalation to triple therapy, switch in treatment, discontinuation of COPD treatment, the end of the study period, or (for the main analyses) 1 year after cohort entry. The treatment segment ended at the earliest of the following events: (1) 15 days after the end of the observed day's supply for the medication received on the index date without a subsequent dispensing of COPD medication (i.e., discontinuation); (2) initiation of triple therapy; or (3) any other change in the use of study medication, including a change to a different combination therapy, from a fixed-form to a free-form combination therapy, or from combination therapy to monotherapy.
Study outcomesThe primary outcome was to compare the risk of first COPD exacerbation after initiating treatment with LABA/LAMA versus LABA/ICS. Exacerbation episodes were identified using outpatient visits with a diagnostic code indicative of a respiratory exacerbation, or receipt of corticosteroids and/or antibiotics used for treating exacerbations, in accordance with previously published algorithms.16,17 To avoid misclassification and over-estimation of exacerbations, consecutive episodes with less than 21 days between prescriptions or GP visits were considered as a single event.
Secondary outcomes included the risk of hospitalization for community-acquired pneumonia, risk of pharmacy dispensation indicating escalation to triple therapy, and risk of any of the following events occurring: COPD exacerbation, hospitalization for pneumonia or escalation to triple therapy. Pneumonia was defined as hospitalization with one of the following diagnostic codes: 481.x–486.x, 487.0, 507.x, 507.0, 507.1, 507.8, 510.0, 510.9, 511.0, 513.0, 514.x, 517.1, 519.8, 530.84 (as per the International Classification of Diseases, version 9, Clinical Modification: ICD-9-CM; used up to October 2015) and J10.0, J11.0, J12-J18, J22, J69, J85.0, J85.1 and J86 (ICD-10; used from October 2015 onwards) (pneumonia without hospitalization was not captured). These definitions of hospitalization data have been used in several previous studies in patients with COPD.21 Escalation to triple therapy was defined as any combination of pharmacy dispensations resulting in the overlapping use of a LAMA, LABA, and ICS through any fixed or free combination for at least 1 day.
Statistical analysisDescriptive analysis was performed with baseline sociodemographic, clinical characteristics and baseline treatments of patients included. Categorical variables were described using absolute frequencies and corresponding percentages. Continuous variables following a normal distribution were described using the mean and standard deviation (SD), while those that did not follow a normal distribution were described using the median and interquartile range.
Time in months between the start of the study treatment and the appearance of the first event (exacerbation, escalation to triple therapy, or any event) was described using the restricted mean survival time (the average time-to-event in a restricted time period) and using Kaplan–Meier analysis over 1 year of follow-up.
The risk of outcomes (exacerbation, escalation to triple therapy, or any event) in patients receiving LABA/ICS versus LABA/LAMA was described using hazard ratios (HRs) with a 95% confidence interval (CI) and p values. Cox proportional hazard models were performed as an as-treated analysis to derive HRs.
Two study sub-cohorts (LABA/LAMA and LABA/ICS) were built based on 1:1 matching through the greedy-matching algorithm (without replacement) and on the propensity score.22 A logistic regression model was used to construct a propensity score that predicts the patient's probability of initiating LABA/LAMA or LABA/ICS treatment. The following variables, included in the model, were collected on the index date: age distribution, sex, time elapsed from COPD diagnosis to the start of treatment, ACO, previous exacerbations and previous treatment billed to compare the evolution at one year of follow-up between cohorts.
All analyses were performed for the whole COPD population and the matched cohort and were also performed according to risk subgroups (high or low risk of exacerbation based on previous exacerbation history and blood eosinophil levels).
All statistical analyses were performed using the statistical software package (SPSS version 20.0, IBM, Chicago, IL, USA).
ResultsStudy populationThe SIDIAP database included 236,162 patients older than 40 years with a diagnosis of COPD; of these, 92,132 (39%) initiated either LABA/LAMA or LABA/ICS during the study period. After excluding those without smoking habits or spirometric confirmation of COPD, the final population of our study was composed of 14,046 confirmed COPD patients; 11,329 (80.6%) initiated LABA/ICS and 2717 (19.4%) LABA/LAMA (Fig. 1).
Patients initiating LABA/ICS were significantly older (69.2 years (SD: 10.4) versus 67.9 (SD: 9.7): p<0.001), were less frequently active smokers (35.5% versus 43.5%; p<0.001), had a higher prevalence of a previous diagnosis of asthma (13.5% versus 4.9%; p<0.001) and had a lower percentage of presenting at least one exacerbation the previous year (70.7% versus 73.3%; p<0.001) while those who had exacerbations presented more episodes (mean number of exacerbations 1.86 (SD: 1.06) versus 1.75 (SD: 0.95); p<0.001). A full description of the characteristics of the study population is shown in Table 1.
Baseline characteristics of the study population in general and according to COPD subcohorts.
| Total n=92,132 | COPD Confirmed n=14,046 | COPD Matched n=3300 | |||
|---|---|---|---|---|---|
| LABA/ICS | LABA/LAMA | LABA/ICS | LABA/LAMA | ||
| n=11,329 | n=2717 | n=1650 | n=1650 | ||
| Sex, men | 62,800 (68.2) | 9489 (83.8) | 2260 (83.2) | 1480 (89.7) | 1480 (89.7) |
| Age; mean (SD) | 71.0 (11.9) | 69.2 (10.4) | 67.9 (9.7)*** | 68.1 (8.8) | 68.1 (8.8) |
| Years from diagnosis | 5.1 (6.6) | 5.6 (6.2) | 4.0 (5.3)*** | 2.7 (3.8) | 2.7 (3.8) |
| BMI, mean (SD) | 28.9 (5.4) | 28.4 (5.1) | 27.8 (5.2)*** | 28.0 (5.1) | 28.3 (5.1) |
| Tobacco | *** | ||||
| Non-smoker | 26,011 (28.2) | ||||
| Smoker | 24,258 (26.3) | 4027 (35.5) | 1183 (43.5) | 675 (40.9) | 709 (43) |
| Former smoker | 40378 (43.8) | 7302 (64.5) | 1534 (56.5) | 975 (59.1) | 941 (57) |
| Unknown | 1485 (1.6) | ||||
| Comorbidities | |||||
| Asthma | 16,483 (17.9) | 1533 (13.5) | 134 (4.9)*** | 14 (0.8) | 14 (0.8) |
| Anxiety | 17,855 (19.4) | 1983 (17.5) | 582 (21.4)*** | 292 (17.7) | 318 (19.3) |
| Depression | 12,455 (13.5) | 1275 (11.3) | 367 (13.5)** | 200 (12.1) | 207 (12.5) |
| Cardiovascular disease | 35,403 (38.4) | 4246 (37.5) | 1074 (39.5)* | 601 (36.4) | 670 (40.6)* |
| Hypertension | 53,634 (58.2) | 6349 (56) | 1527 (56.2) | 933 (56.5) | 927 (56.2) |
| Dyslipidaemia | 43,190 (46.9) | 5582 (49.3) | 1369 (50.4) | 409 (24.8) | 417 (25.3)* |
| Complementary tests | |||||
| FEV1(%), mean (SD) | 70.9 (15.1) | 63.6 (12.3) | 64.6 (11.1)*** | 64.2 (11.4) | 64.6 (10.9) |
| Stage N (%) | ** | ||||
| Gold I | 7138 (29.2) | 184 (1.6) | 38 (1.4) | 22 (1.3) | 20 (1.2) |
| Gold II | 15,181 (62) | 9598 (84.7) | 2379 (87.6) | 1420 (86.1) | 1458 (88.4) |
| Gold III | 1990 (8.1) | 1432 (12.6) | 282 (10.4) | 202 (12.2) | 162 (9.8) |
| Gold IV | 171 (0.7) | 115 (1) | 18 (0.7) | 6 (0.4) | 10 (0.6) |
| Blood eosinophil, mean (SD) | 268.9 (264.9) | 277.2 (305.8) | 275.6 (297.5) | 282.1 (237.3) | 276.5 (331.2) |
| Use of health resources | |||||
| Exacerbations | 59,143 (64.2) | 8009 (70.7) | 1992 (73.3)*** | 1317 (79.8) | 1317 (79.8) |
| Exacerbations, mean (SD) | 1.82 (1.05) | 1.86 (1.06) | 1.75 (0.95)*** | 1.81 (1.01) | 1.73 (0.93)* |
| 0 exacerbations | 32,989 (35.8) | 3320 (29.3) | 725 (26.7) | 333 (20.2) | 333 (20.2) |
| 1 exacerbation | 29,668 (32.2) | 3869 (34.2) | 1024 (37.7) | 647 (39.2) | 690 (41.8) |
| ≥2 exacerbations | 29,475 (32) | 4140 (36.5) | 968 (35.6) | 670 (40.6) | 627 (38) |
| Admissions | 761 (0.8) | 78 (0.7) | 30 (1.1)* | 9 (0.5) | 21 (1.3)* |
Footnote: Data are n (%). BMI: body mass index; COPD: chronic obstructive pulmonary disease; ICS: inhaled corticosteroid; LABA: long-acting beta-2 agonist; LAMA: long-acting antimuscarinic agent; FEV1: forced expiratory volume in one second; GOLD: global initiative for obstructive lung disease; SD: standard deviation; BMI, body mass index.
After matching for the most important variables related to the risk of exacerbations, the matched population consisted of 1650 patients per treatment arm (Table 1).
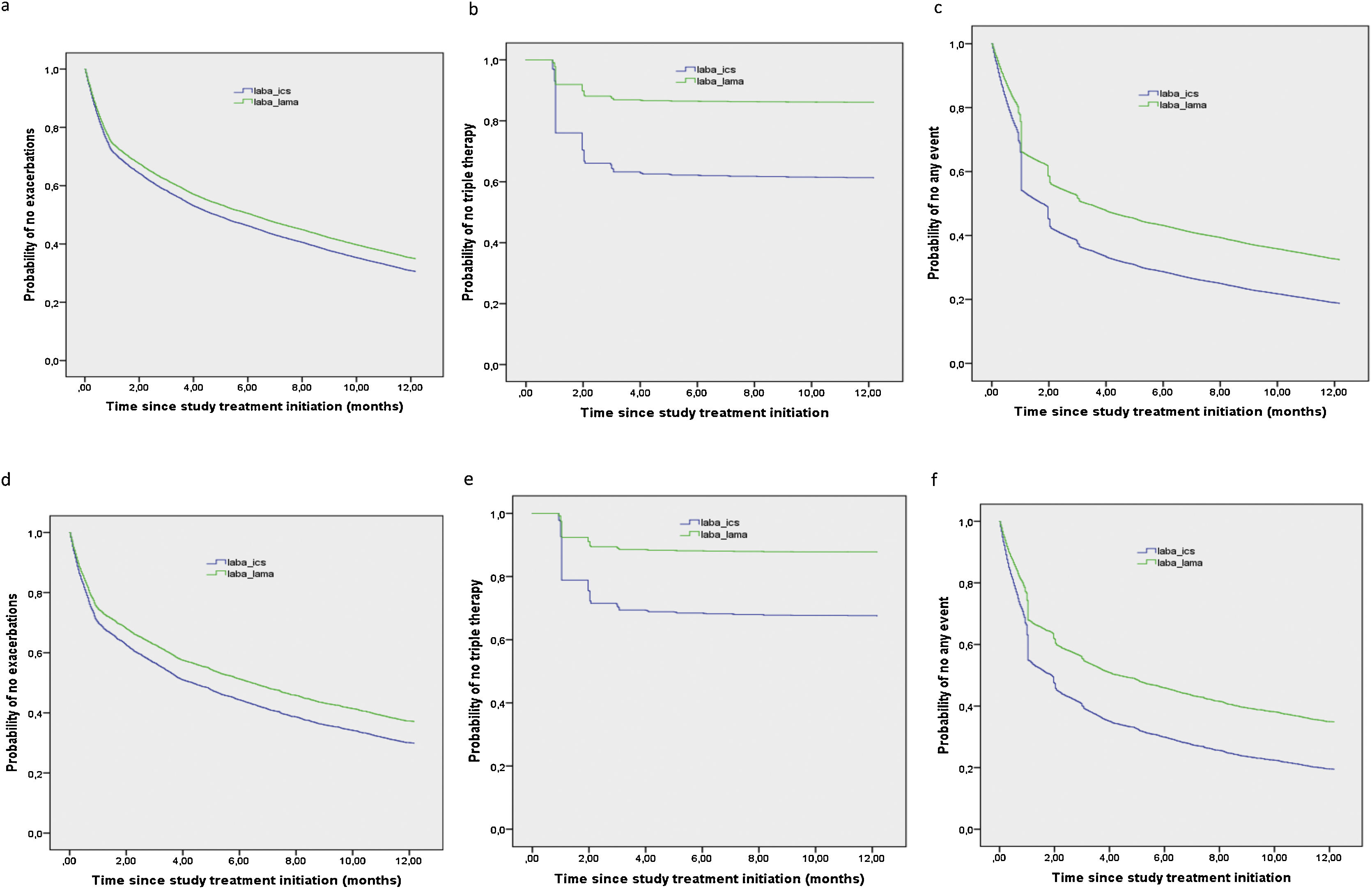
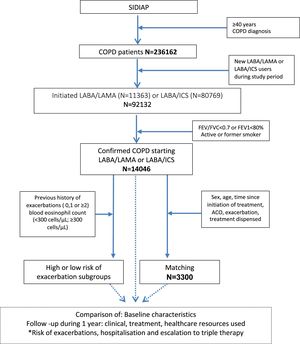
Main outcome: time to first exacerbation, hospitalization or escalation to triple therapyDuring the one-year follow-up, 68.5% of patients in the global population experienced an exacerbation (69.6% in the LABA/ICS group and 64.4% in the LABA/LAMA group). The mean time to the first exacerbation was 6.03 months (95%CI: 5.94–6.12) for LABA/ICS and 6.4 months (95%CI: 6.21–6.59) for LABA/LAMA; p<0.001. The time to escalation to triple therapy and to the combination of all events was also significantly prolonged in LABA/LAMA compared to LABA/ICS patients (Table 2, Fig. 2a–c). Differences were only non-significant for hospitalization due to the extremely small number of events (29 (0.21%) for LABA/ICS and 12 (0.08%) for LABA/LAMA).
Time in months between the start of the study treatment and the appearance of the first event according to COPD subgroups (COPD confirmed and COPD matched cohorts).
| COPD confirmed | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| COPD confirmed N=14,046 | LABA/ICS N=11,329 | LABA/LAMA N=2717 | |||||||||||
| N | % | Restricted mean | 95% lower upper limit | N | % | Restricted mean | 95% lower upper limit | N | % | Restricted mean | 95% lower upper limit | P | |
| Exacerbations | 9631 | 68.5 | 6.10 | 5.94; 6.12 | 7882 | 69.6 | 6.03 | 5.94; 6.12 | 1749 | 64.4 | 6.40 | 6.21; 6.59 | 0.001 |
| Triple therapy | 4772 | 34 | 8.06 | 8.5; 8.6 | 4410 | 39 | 8.09 | 7.9; 8.1 | 2355 | 13.3 | 10.75 | 10.62; 10.89 | 0.001 |
| Any event | 11,042 | 78.6 | 4.47 | 4.40; 4.55 | 9219 | 81.4 | 4.13 | 4.05; 4.21 | 1823 | 67.1 | 5.91 | 4.20; 5.72 | 0.001 |
| COPD matched cohorts | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| COPD matched N=3300 | LABA/ICS N=1650 | LABA/LAMA N=1650 | |||||||||||
| N | % | Restricted mean | 95% lower upper limit | N | % | Restricted mean | 95% lower upper limit | N | % | Restricted mean | 95% lower upper limit | P | |
| Exacerbations | 2194 | 66.5 | 6.21 | 6.03; 6.38 | 1158 | 70.5 | 5.85 | 5.61; 6.11 | 1036 | 62.8 | 6.56 | 6.31; 6.80 | 0.001 |
| Triple therapy | 741 | 22.5 | 9.81 | 9.66; 9.96 | 545 | 33 | 8.72 | 8.48; 8.96 | 196 | 11.9 | 10.90 | 10.73; 11.07 | 0.001 |
| Any event | 2401 | 72.8 | 5.17 | 5; 5.34 | 1328 | 80.5 | 4.21 | 3.98; 4.43 | 1073 | 65 | 6.13 | 5.88; 6.37 | 0.001 |
Footnote: COPD: chronic obstructive pulmonary disease; ICS: inhaled corticosteroid; LABA: long-acting beta-2 agonist; LAMA: long-acting antimuscarinic agent.
Kaplan–Meier curves for cumulative incidence of the first event. a–c in the COPD confirmed population: (a) time to the first moderate/severe exacerbation; (b) time to the escalation to triple therapy; (c) time to the combined event. d–f in the matched cohort: (d) time to the first moderate/severe exacerbation; (e) time to the escalation to triple therapy; (f) time to the combined event.
These results were replicated in the matched cohort, in whom time to the first exacerbation, time to escalation to triple therapy and time to the combined event were significantly prolonged in patients initiating LABA/LAMA compared to those initiating LABA/ICS (p<0.001 for all three comparisons, Table 2 and Fig. 2d–f). The number of hospitalisations for pneumonia was very small, and the differences were not significant (6 (0.4%) for LABA/ICS and 10 (0.6%) for LABA/LAMA).
Time to first exacerbation or escalation to triple therapy by subgroupsThe results in terms to time to the next exacerbation, time to escalation to triple therapy or both combined were very similar among the different subgroups of patients classified according to the frequency of previous exacerbations and blood eosinophil levels. Due to the small numbers of hospitalization for pneumonia by subgroups (between 2 and 7 in the whole population and between 0 and 3 in the matched cohort) no statistical comparisons were conducted for this variable. In all cases, the time to the next event was numerically superior for LABA/LAMA versus LABA/ICS and the differences were statistically significant for the time to triple therapy and the time to the next event in all four subgroups with low and high risk of exacerbations and high and low blood eosinophil counts. The time to the next exacerbation was significantly longer for LABA/LAMA users in patients with infrequent exacerbations and high blood eosinophils (7.2 months, 95%CI: 6.6–7.6, versus 6.8, 95%CI: 6.5–7.0; p<0.05) and in patients with frequent exacerbations and low blood eosinophil counts (5.2, 95%CI: 4.8–5.6, versus 4.5, 95%CI: 4.3–4.7; p<0.01) (Table 3).
Time in months between the start of the study treatment and the appearance of the first event in patients according to risk subgroups and according to COPD subgroups (COPD confirmed and COPD matched cohorts).
| COPD confirmed | ||||||||
|---|---|---|---|---|---|---|---|---|
| Low risk of exacerbations | High risk of exacerbations | |||||||
| Eosinophils<300cel/μLN=4368 | Eosinophils≥300cel/μLN=1997 | Eosinophils<300cel/μLN=2585 | Eosinophils≥300cel/μLN=1213 | |||||
| LABA/ICSN=3502 (80.2) | LABA/LAMAN=866 (19.8) | LABA/ICSN=1606 (80.4) | LABA/LAMAN=391 (19.6) | LABA/ICSN=2083 (80.6) | LABA/LAMAN=502 (19.4) | LABA/ICSN=967 (79.7) | LABA/LAMAN=246 (20.3) | |
| ExacerbationsRestricted mean; (95%lower upper limit) | N=22396.65(6.49;6.82) | N=5326.78(6.44;7.11) | N=10036.81 (6.57;7.04) | N=2177.17(6.67;7.66)* | N=17084.57(4.37;4.76) | N=3785.23 (4.83;5.67)** | N=7575.02 (4.72;5.31) | N=1895.36(4.77;5.95) |
| Triple therapyRestricted mean; (95%lower upper limit) | N=13108.26(8.09;8.43) | N=9311.03(10.81;11.25)*** | N=5928.29 (8.04;8.54) | N=5010.80 (10.44;11.15)*** | N=8208.01(7.79;8.23) | N=6610.76 (10.45;11.08)*** | N=3947.94 (7.61;8.27) | N=519.97 (9.43;10.51)*** |
| Any eventRestricted mean; (95%lower upper limit) | N=27114.62(4.46;4.78) | N=5576.33(5.99;6.67)*** | N=12254.48 (4.54;5.02) | N=2356.51(6.01;7.03)*** | N=18423.19(3.02;3.36) | N=3854.90(4.85; 5.32)*** | N=8383.46 (3.20;3.72) | N=1944.81 (4.22;5.40)*** |
| COPD matched cohorts | ||||||||
|---|---|---|---|---|---|---|---|---|
| Low risk of exacerbations | High risk of exacerbations | |||||||
| Eosinophils<300N=988 | Eosinophils≥300N=470 | Eosinophils<300N=661 | Eosinophils≥300N=N=309 | |||||
| LABA/ICSN=469 (47.5) | LABA/LAMAN=519 (52.5) | LABA/ICSN=247(52.6) | LABA/LAMAN=223(47.4) | LABA/ICSN=340(51.4) | LABA/LAMAN=321(48.6) | LABA/ICSN=147(47.6) | LABA/LAMAN=162(52.4) | |
| ExacerbationsRestricted mean; (95%lowerupper limit) | N=3186.11 (5.66;6.57) | N=3196.73 (6.31;7.17)* | N=1526.69 (6.05;7.33) | N=1187.39(6.74;8.05) | N=2655.10 (4.60;5.60) | N=2305.63(5.09;6.17) | N=1115.11 (4.31;5.91) | N=1175.77(5.02;6.53) |
| Triple therapyRestricted mean; (95%lowerupper limit) | N=1518.84(8.41;9.29) | N=4611.23(10.97;11.50)*** | N=719.14 (8.54;9.74) | N=2410.99 (10.54;11.43)*** | N=1019.01 (8.49;9.53) | N=4110.82 (10.4;11.21)*** | N=568.28 (7.49;9.11) | N=3110.18 (9.54;10.82)*** |
| Any eventRestricted mean; (95%lowerupper limit) | N=3604.59 (4.15;5.03) | N=3286.43 (5.99;6.87)*** | N=1864.81 (4.20;5.41) | N=1286.76 (6.09;7.43)*** | N=2853.91(3.43; 4.37) | N=2355.28(4.74; 5.82)*** | N=1253.47 (2.77;4.16) | N=1215.22 (4.46;5.97)*** |
Footnote: COPD: chronic obstructive pulmonary disease; ICS: inhaled corticosteroid; LABA: long-acting beta-2 agonist; LAMA: long-acting antimuscarinic agent.
Similarly, for patients in the matched cohorts, the time to the next event was always numerically longer in the LABA/LAMA compared to the LABA/ICS group and differences were highly significant for time to escalation to triple therapy and combined outcome for the four subgroups (p<0.001 for all comparisons) and significant with p<0.05 for the time to the next exacerbation in the subgroup of patients with infrequent exacerbations and low blood eosinophils (Table 3).
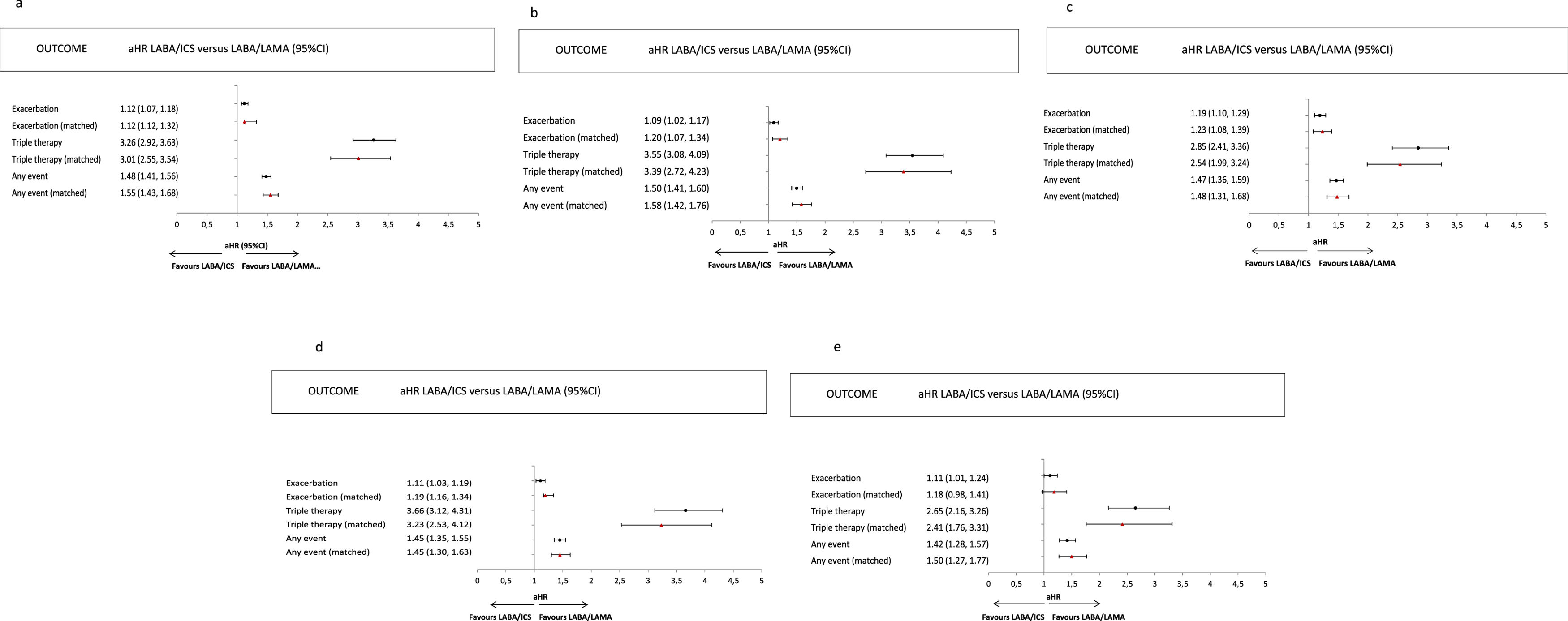
Risk of outcomesThe risk of exacerbations was higher for LABA/ICS users (adjusted HR [aHR]: 1.12, 95%CI: 1.07–1.18). Similarly, the risk of escalation to triple therapy (aHR: 3.26, 95%CI: 2.92–3.63) and the risk of combined outcome (aHR: 1.48, 95%CI: 1.41–1.56) were also significantly higher for patients treated with LABA/ICS (Fig. 3a).
Risk of exacerbation, escalation to triple therapy, or any event in patients receiving LABA/ICS versus LABA/LAMA for the whole COPD population and the matched cohort. (a) The global population; (b) patients at low risk of exacerbations; (c) patients at high risk of exacerbations; (d) patients with low blood eosinophil counts; (e) patients with high blood eosinophil counts. Footnote: Hazard ratios were derived using Cox proportional hazard models. aHR: adjusted hazard ratio; CI: confidence interval; COPD: chronic obstructive pulmonary disease; ICS: inhaled corticosteroid; LABA: long-acting beta-2 agonist; LAMA: long-acting antimuscarinic agent.
Similar results were observed for patients stratified by the frequency of baseline exacerbations and for patients with eosinophil levels lower or higher than 300cells/μL. Only in the subgroup of patients with more than 300eosinophils/μL were no significant differences in the risk of exacerbations observed between the two treatment groups (Fig. 3b–e).
The analysis in the matched cohort showed similar results. The aHR estimates varied from 1.18 to 3.39 for risk of exacerbation, escalation to triple therapy or the combined outcome, and the results were non-significant only for the risk of exacerbation in patients with more than 300eosinophils/μL (Fig. 3a–e).
DiscussionThis large database study, which is representative of the general population of patients with COPD in primary care, has shown that initiating treatment with a combination of LABA/LAMA compared to LABA/ICS results in a prolonged time to the next exacerbation or escalation to triple therapy. These benefits persist in a well-matched population according to the recognized risk factors for exacerbations and in different subgroups of patients classified by the previous frequency of exacerbations and their blood eosinophil counts. Differences in the time to the first exacerbation were not statistically significant only in patients with frequent exacerbations and more than 300eosinophils/μL. These results suggest that in the majority of cases dual bronchodilation should be the preferred option over LABA/ICS for the initial therapy of symptomatic patients with COPD in primary care, as recommended by guidelines.
Randomized clinical trials comparing the efficacy and safety of LABA/LAMA versus LABA/ICS have demonstrated superior bronchodilation and control of symptoms with dual bronchodilation.23–25 Moreover, the combination of indacaterol/glycopyrronium was more effective than salmeterol/fluticasone in the prevention of exacerbations in the FLAME trial,7 irrespective of the blood eosinophil concentrations of the participants.8 However, recent large clinical trials of triple therapy (LABA/LAMA/ICS) versus LABA/LAMA or LABA/ICS (IMPACT and ETHOS trials) observed a reduced risk of exacerbations with the LABA/ICS compared with LABA/LAMA combination.9,10 These contradictory results may be explained by the differences in the characteristics of the patients included in these trials. Only 20% of patients included in the FLAME study had more than one exacerbation in the previous year,7 while around 55% of patients in ETHOS and IMPACT studies had two or more exacerbations and the remaining patients had at least one hospital admission or moderate exacerbation, if they had an FEV1(%)<50%, in the previous year.9,10 These data suggest that ICS may be more effective in the prevention of exacerbations in more severe COPD patients and in those more at risk of exacerbations,26,27 while milder patients with infrequent exacerbations may obtain greater benefit from treatment with bronchodilators alone without the unnecessary risks associated with the long-term use of ICS.14
These conclusions are very relevant for clinical practice because the majority of patients in primary care in different countries are infrequent exacerbators. In our global population of +90,000 patients, only one third had two or more exacerbations and less than 1% had had a hospital admission in the previous year. In the recent NOVELTY international multicentre study including almost 4000 patients with COPD, half of whom were recruited from teaching hospitals and half from primary care, only 5.3% of mild, 9.5% of moderate and 21.7% of severe patients presented more than one exacerbation in the previous year.28
Our results concur with those obtained in previous database studies conducted in other countries. Suissa et al.29 compared the results obtained in 1977 initiators of LABA/LAMA matched to the same number of initiators of LABA/ICS in the UK Clinical Practice Research Datalink; their results showed no difference in the HR of moderate to severe exacerbations for both treatments, but a significantly lower incidence of pneumonia requiring hospitalization with LABA/LAMA (HR: 0.66).29 Another study with data from a US health insurer database showed an adjusted 30% on treatment reduced risk of a first moderate or severe exacerbation and a 35% adjusted reduced risk of escalation to multiple-inhaler triple therapy in patients treated with LABA/LAMA (umeclidinium/vilanterol) compared with LABA/ICS (fluticasone/salmeterol).30 Finally, a recent study using data from the Health Core Integrated Research Database in the US demonstrated a 24% adjusted reduction in the risk of exacerbations and 78% adjusted reduction in the escalation to triple therapy with LABA/LAMA (tiotropium/olodaterol) compared with LABA/ICS.11
The results obtained from clinical trials and observational studies are consistent in showing a higher efficacy of ICS in the prevention of exacerbations of COPD in patients with more frequent exacerbations and higher blood eosinophil counts9,10,26,27,31; however, our results showed a reduced risk of exacerbations and escalation to triple therapy with LABA/LABA compared with LABA/ICS, irrespective of these two variables. Even in the group of patients with more than one exacerbation in the previous year and more than 300eosinophils/μL, the time to escalation to triple therapy and the time to any event were significantly longer for patients initiating LABA/LAMA, although no significant differences were observed in the time to the first exacerbation, in both the COPD population in general and the matched cohort. Our results could be compared with a subgroup analysis of the IMPACT trial. Han et al.32 investigated the possible effect of baseline medications on the results of the study and observed that there were no differences in the risk of exacerbations (relative risk [RR]: 0.99, 95%CI: 0.71–1.39; p=0.964) between LABA/LAMA and triple therapy (i.e., with the addition of an ICS) in patients who escalated from a LAMA or, in other words, in initiators of LABA/LAMA versus initiators of triple therapy. These results again support the recommendation to initiate LABA/LAMA instead of LABA/ICS or before escalation to triple therapy.
There is a growing number of patients who are initiated on ICS in primary care without fulfilling the criteria recommended in guidelines.12,33 A population-based study in Spain showed that between 43.8% of non-exacerbator COPD patients in 2007 and 35.8% in 2012 initiated treatment with ICS in different combinations.16 More recently, our group showed that among +34,000 patients with COPD initiating triple therapy in primary care between 2011 and 2015, only 16.5% were frequent exacerbators and 5.6% were diagnosed with ACO17,20; therefore, no clear indication for ICS could be established in the remaining 77.9% of cases.17
Our study has some limitations. Although the results were consistent in the whole group of COPD and in the matched cohort, we cannot rule out the impact of some unmeasured confounders. However, unlike most studies conducted with primary care databases, in the analysis we only included patients with spirometric results in order to minimize the effect of a possible disbalance in severity between groups. Although we used a validated algorithm to identify exacerbations, the risk of underreporting still exists and underreporting of exacerbations by patients is also well documented. In any case, if any underreporting were present, it would affect all subgroups equally and would not significantly affect the conclusions of the study. However, we must consider that clinicians do not select treatment randomly, and patients treated with ICS may have a higher risk profile. The type of analysis conducted in this study takes into account most known risk factors for exacerbations, but it can’t rule out the effect of unknown sources of bias.
ConclusionsPatients initiating LABA/LAMA treatment in primary care had a significantly prolonged time to the first exacerbation and to escalation to triple therapy compared to patients initiating LABA/ICS. These results were not clearly influenced by a previous history of exacerbations or blood eosinophil levels. Differences in the time to the first exacerbation were not significant only in the subgroup of patients more likely to respond to ICS (i.e., those with frequent exacerbations and more than 300eosinophils/μL). Our results support the current recommendations to initiate treatment with LABA/LAMA before introducing an ICS or escalate to triple therapy.
FundingThis study was funded by Boehringer Ingelheim S.A.
Conflict of interestMònica Monteagudo has no conflicts to disclose. Alexa Núñez has received speaker fees from GlaxoSmithKline and consulting fees from CSL Behring. Miriam Barrecheguren has received speaker fees from Grifols, Menarini, CSL Behring, GSK, Boehringer Ingelheim and consulting fees from GSK, Novartis, CSL Behring and Boehringer Ingelheim. Marc Miravitlles has received speaker fees from AstraZeneca, Boehringer Ingelheim, Chiesi, Cipla, Menarini, Rovi, Bial, Sandoz, Zambon, CSL Behring, Grifols and Novartis, consulting fees from AstraZeneca, Atriva Therapeutics, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Bial, Gebro Pharma, CSL Behring, Laboratorios Esteve, Ferrer, Mereo Biopharma, Verona Pharma, Spin Therapeutics, ONO Pharma, pH Pharma, Palobiofarma SL, Takeda, Novartis, Sanofi and Grifols and research grants from Grifols.