The latest SEPAR guidelines published in 20171 define bronchiectasis as a chronic inflammatory bronchial disease that involves irreversible dilation of the bronchial lumen due to different causes. The conventional notion of bronchiectasis is grounded on the concept of permanent damage to the bronchial lumen. However, the recent development of new treatments to correct protein deficiencies in cystic fibrosis, such as CFTR modulators, has led to significant improvements in patients’ lung function and the resolution of cough and bronchorrhea, the main symptoms of bronchiectasis. Radiological improvements are also being observed in chest computed tomography (CT), some of which are striking, as in the case described below.
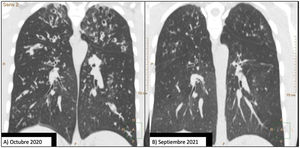
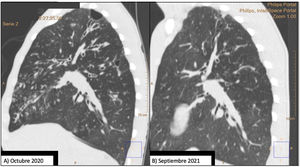
Our patient was a 20-year-old man with cystic fibrosis (F508del/I507del), advanced lung disease, and chronic bronchial infection with Staphylococcus aureus and Pseudomonas aeruginosa, treated with nebulized ampicillin and colistin, respectively. He was also receiving treatment to assist with bronchial drainage, including hypertonic saline and dornase alfa. He was on the waiting list for lung transplantation due to frequent, occasionally serious, respiratory exacerbations and very severe airflow obstruction, with a forced expiratory volume in 1 second (FEV1) of 1100ml (25%). Chest CT performed in October 2020 (Figs. 1A and 2A) showed extensive bilateral severe bronchiectasis, mucus plugging, tree-in-bud pattern, and significant thickening of the bronchial walls, corresponding to a modified Bhalla score of 11. This radiological score is graded from 0 to 25; the lower the score, the higher the radiological severity.2
Comparison of 2 coronal chest CT reconstructions. Image (A) shows extensive bilateral bronchiectasis, with significant thickening of the bronchial walls, mucus plugging, and a tree-in-bud pattern. After 1 year of treatment with elaxacaftor/tezacaftor/ivacaftor, image (B), bronchiectasis is significantly reduced and practically resolved.
Comparison of 2 sagittal chest CT reconstructions. Image (A) shows a thin-walled cystic lesion (bulla), extensive bilateral bronchiectasis, with significant thickening of the bronchial walls and a tree-in-bud pattern. After 1 year of treatment with elaxacaftor/tezacaftor/ivacaftor, image (B), bronchiectasis is significantly improved and the apical bulla is resolved.
Given the patient's clinical situation, he was enrolled in an early access program for treatment with CFTR modulators, a compassionate use program known as MyMAPs. In October 2020, treatment began with elexacaftor/tezacaftor/ivacaftor (ETI) 75/50/100mg, 2 tablets at breakfast and 1 tablet of ivacaftor 150mg at dinner. After 1 year of follow-up, the patient had improved significantly. Lung function had increased by 25% (FEV1: 2450ml, 55.8%), respiratory exacerbations had dramatically reduced – no events since the start of treatment – cough and bronchorrhea had resolved, and exercise tolerance had improved. Follow-up chest CT showed a significant improvement in mucus plugging, a significant reduction in the size and number of bronchiectasis, some of which had disappeared (Fig. 1B), and a reduction in the size of the bulla in the right upper apex (Fig. 2B). The Bhalla score increased from 6 to 17 points in September 2021. The patient was therefore taken off the lung transplantation waiting list.
This case challenges the notion of irreversible bronchial damage, at least in the case of bronchiectasis.
Indeed, some authors, particularly those who see pediatric patients, are keen to abandon the notion of irreversible3 from the definition of bronchiectasis. They also recommend adjusting the bronchoarterial ratio for radiological diagnosis, since the diagnostic criteria were extrapolated from adult data, despite the fact that lung volume increases with time.4 Bush et al.4 and Gaillard et al.5 reported that mild and cylindrical bronchiectasis in early life could be reversed if the condition is investigated and treated promptly with targeted management. They also proposed that the diagnosis of bronchiectasis should not be merely radiological, but should also be supported by a consistent clinical picture. Furthermore, Martinez et al.6 coined the term “bronchiectasis syndrome” to encompass the various bronchiectasis phenotypes and reflect the complexity and heterogeneity of this disease.
Cole7 and others report that a poorly placed chest tube can foster bacterial colonization and the subsequent release of proinflammatory mediators that will lead to tissue damage and the development of bronchiectasis. To date, treatments such as chronic administration of macrolides, mucolytic substances and antibiotics have been used to break this vicious cycle. However, highly effective treatments are now available that restore bronchial drainage levels to near normal, that, when administered promptly in early life, may prevent the tissue damage caused by the subsequent appearance of bronchiectasis.
As early as 2020, Chalmers et al. published the results of a phase 2 clinical trial of brensocatib, an oral reversible inhibitor of dipeptidyl peptidase 1 (DPP-1), an enzyme responsible for the activation of neutrophil serine proteases.8 Treatment prolonged the time to the first exacerbation and reduced the number of these events with an adequate safety profile. The study is currently in an expanded access phase.9
The striking feature of our clinical case is that the patient is a young adult who had severe, established bronchial damage, with cylindrical, cystic and string-of-pearls bronchiectasis in all lung lobes, in addition to chronic Pseudomonas aeruginosa bronchial infection, factors that would have a negative impact on disease progression and survival. Despite this, the patient's clinical, functional and radiological progress with ETI has been very favorable.
ETI, approved in Spain in December 2021, is indicated for patients with cystic fibrosis with at least one F508del mutation, explaining the lack of literature on radiological changes observed with this novel treatment. Some recent studies in cystic fibrosis have echoed the significant functional and radiological improvement achieved with ETI therapy.
Wucherpfennig et al. described a significant improvement in sinus and bronchial abnormalities detected on magnetic resonance imaging in patients taking the same CFTR modulator as our patient. A retrospective, controlled, observational study found a significant reduction in bronchiectasis, bronchial wall thickening, and mucus plugging, as well as a reduction in maxillary and ethmoid sinus mucoceles in the ETI group.10 A study published by Beswick et al. reported significant improvements in CT imaging in cystic fibrosis patients with chronic rhinosinusitis treated with ETI, a finding that was also reflected in quality of life.11
We therefore believe that it is appropriate to continue evaluating and studying the reversibility of bronchiectasis, and perhaps even to consider dropping the term irreversible from the definition.