Nontuberculous mycobacteria (NTM) are classified by their growth rate, either slowly growing or rapidly growing. Rapidly growing mycobacteria (RGM) produce mature colonies on agar plates within 7 days.1 They have a special ability to create a biofilm, which enhances a catheter-related bloodstream infection.2 Furthermore, RGM induce skin and soft tissue infections, osteomyelitis, and pulmonary infections.2 The most commonly encountered RGM are Mycobacterium abscessus complex, M. chelonae, and M. fortuitum complex.3M. mucogenicum group, which comprises M. mucogenicum, M. aubagnense, and M. phocaicum, is another set of RGM.3 A 16S rRNA gene sequence analysis helps in discriminating between M. mucogenicum and M. aubagnense.4 Furthermore, M. phocaicum and M. mucogenicum can be discriminated by rpoB gene and heat-shock protein (hsp)-65 gene sequence analysis.4 In this report, we describe the case of a patient with pulmonary M. mucogenicum and M. phocaicum infection, whose gallium-67 (67Ga) scintigraphy reveals diffuse pulmonary uptake without any abnormal findings on chest computed tomography (CT) scan.
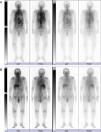
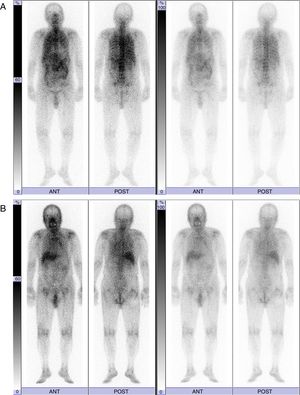
We report the case of an 88-year-old non-smoking male patient who was diagnosed with hypertension. He was referred to Hikone Municipal Hospital because of malaise lasting for approximately 1 week. While he did not present with a fever or any respiratory symptoms, he complained of spontaneous pain in the right scapula. He had neither used any humidifiers nor hot tubs. His white blood cell (WBC) count was 12610/μl and C-reactive protein level (CRP) was 17.49mg/dL. While chest X-ray and chest CT scan revealed no remarkable changes, 67Ga scintigraphy demonstrated diffuse pulmonary uptake (Fig. 1A). Although the induced sputum culture tested negative for bacteria, it gave positive results for mycobacteria. Bone marrow aspiration analysis and biopsy revealed a slightly hypocellular-to-normocellular bone marrow, and the bone marrow culture tested negative for bacteria and acid-fast bacillus. Because we initially suspected miliary tuberculosis, the antituberculosis drug isoniazid (300mg/day) and rifampicin (450mg/day) were administered. We conducted the bronchoscopic examination and prescribed levofloxacin (500mg/day) 1 week after the administration of antituberculosis drugs because the patient had taken aspirin (an antiplatelet drug). Transbronchial lung biopsy did not detect any malignant tumors and granulomas. While the bronchial lavage culture tested negative for bacteria, it was positive for mycobacteria. Reportedly, the WBC count and CRP level decreased to 6710/μl and 3.09mg/dL, respectively, 2 weeks after the antituberculosis regimen. After 3 months of the antituberculosis regimen, mycobacteria cultured from the induced sputum using a DNA–DNA hybridization method did not identify any particular species. However, M. mucogenicum and M. phocaicum were identified using 16S rRNA gene, rpoB gene, and hsp-65 gene sequence analyses. Therefore, we changed the treatment to clarithromycin (600mg/day). At that time, 67Ga scintigraphy demonstrated decreased pulmonary uptake and equivocal pulmonary uptake (Fig. 1B). In addition, the WBC count and CRP level declined to 4690/μl and 0.26mg/dL, respectively. Three months after the administration of clarithromycin, 67Ga scintigraphy revealed no changed in equivocal pulmonary uptake, and the WBC count and CRP level decreased to 5450/μl and 0.22mg/dL, respectively.
This case is interesting from three viewpoints. First, 67Ga scintigraphy helped to resolve the inflammatory condition of unknown etiology. Second, diffuse pulmonary uptake in 67Ga scintigraphy was induced by pulmonary M. mucogenicum and M. phocaicum infection. Finally, we could monitor the treatment responses using serial 67Ga scintigraphy.
67Ga accumulates in inflammatory and infection sites by increased vascular membrane permeability and binding transferrin, lactoferrin, and siderophores.5 Therefore, 67Ga scintigraphy guides to a physician to a fertile site for additional investigation in patients with a fever of unknown origin.6 In our case, 67Ga scintigraphy helped in resolving the inflammatory condition of unknown etiology.
The patterns of 67Ga uptake in the thorax include (a) normal uptake, (b) lymph node uptake, (c) focal pulmonary parenchymal uptake, and (d) diffuse pulmonary parenchymal uptake. Of these, diffuse pulmonary parenchymal uptake of 67Ga indicates Pneumocystis jiroveci pneumonia, miliary tuberculosis, interstitial pneumonitis, drug-induced pneumonitis, and hypersensitivity pneumonitis.5,7–9 In addition, 67Ga scintigraphy can be used to monitor the therapy response in patients with P. jiroveci pneumonia and miliary tuberculosis.5,7 In this case, the presence of M. mucogenicum and M. phocaicum in the sputum and bronchial lavage and diffuse pulmonary uptake in 67Ga scintigraphy lead to a differential diagnosis of NTM-induced hypersensitivity pneumonitis. However, the patient had neither used any humidifiers nor hot tubs. Furthermore, chest CT revealed no remarkable changes. Therefore, the possibility of NTM-induced hypersensitivity pneumonitis was low in this case. Nevertheless, in the future, we need to carefully investigate the disease profile of this case because not much is known about the disease profiles of patients with NTM disease and diffuse pulmonary parenchymal uptake in 67Ga scintigraphy.
Reportedly, M. mucogenicum and M. phocaicum are susceptible to amikacin, clarithromycin, imipenem, trimethoprim-sulfamethoxazole, and linezolid.2,10 In our case, the antituberculosis treatment with isoniazid, rifampicin, and levofloxacin for 3 months improved the inflammatory condition and abnormal finding of 67Ga scintigraphy. After this treatment, we changed the antimicrobial regimen to clarithromycin according to the antimicrobial susceptibility described above, although we did not test the antimicrobial susceptibility. In general, the use of combination antimicrobial regimens is superior to monotherapy and tends to be associated with a lower relapse rate.2 Here, we selected monotherapy because the subject was an elderly patient. In the future, further investigation of this disease profile is required because the efficacy of clarithromycin monotherapy for patients with pulmonary M. mucogenicum and M. phocaicum infection is not yet convincing.
Although 18F-fluorodeoxyglucose positron emission tomography is a promising technique for diagnosing infection and inflammation of unknown etiologies, it has disadvantages of limited availability and high cost.7 Therefore, 67Ga scintigraphy remains a widely used technique for radiopharmaceutical diagnostic imaging.7 In our case, we could solve the inflammatory condition of unknown etiology and monitor the treatment responses using 67Ga scintigraphy.
The authors thank Ms. Yuko Kazumi (The Research Institute of Tuberculosis, Japan Anti-Tuberculosis Association) for performing 16S rRNA gene, rpoB gene, and hsp-65 gene sequence analysis.