The outbreak of COVID-19 caused by the novel coronavirus represents the most severe global epidemic in the last three years, resulting in millions of deaths, significant adverse effects on human health, and considerable disruptions to economic and social development.1–4 The main symptoms of COVID-19 are fever, cough, fatigue, slight dyspnoea, sore throat, headache, conjunctivitis and gastrointestinal issues. The disease mainly transmits through respiratory droplets and close contact, with the general population being susceptible to the virus.5 The second wave of the pandemic saw the emergence of the ‘Delta’ variant of SARS-CoV2, which has a higher mortality rate than the original strain. Meanwhile, the third wave was driven by the less virulent but more transmissible variant named ‘Omicron’.6 Due to its high transmissibility, the virus has undergone several mutations that have led to the emergence of multiple variants. Currently, the most prevalent circulating strains include BA.5.2, CH.1.1, BQ.1, and XBB, whereas no studies on the clinical characteristics and disease outcomes of patients with XBB and BA.5.2. Therefore, we conducted a retrospective analysis of 119 patients who were infected with XBB and BA.5.2 from the First Affiliated Hospital of Sun Yat-sen University between December 2022 and July 2023, and compared the similarities and differences.
The clinical manifestations of patients with XBB and BA.5.2 (SARS-COV2 subtype was tested by Geneplus-Beijing Institute) were mainly in the respiratory system (61% and 78%, respectively). Notably, the digestive system manifestations of XBB patients were also relatively common, accounting for 17% of cases. Among the 119 patients included in the study, 36 had a history of at least one pre-existing malignancy (26 for XBB and 10 for BA.5.2), and 18 had received organ transplants (8 for XBB and 10 for BA.5.2).
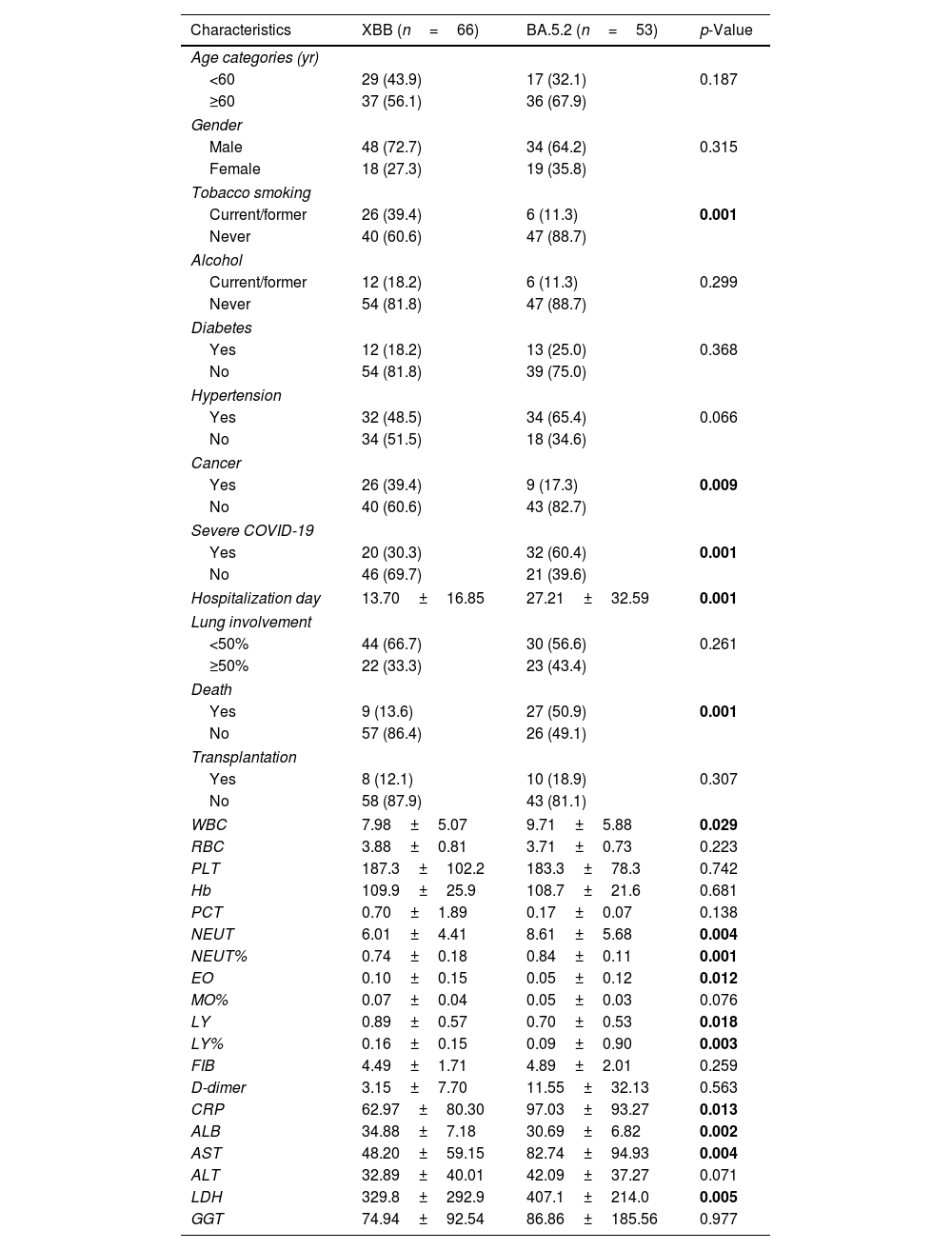
As shown in Table 1, the prevalence of cancer in XBB patients was much higher than BA.5.2 patients (39.4% for XBB versus 17.3% for BA.5.2, p=0.009), whereas there were no significant differences in other underlying diseases such as hypertension and diabetes mellitus between the two SARS-COV2 subtypes. The incidence of critical patients with BA.5.2 infection was much higher than XBB infection (60.4% versus 30.3%, p=0.001). Notably, the mortality rate was more than three times higher for patients with BA.5.2 strains than for those with XBB strains (50.9% versus 13.6%, p=0.001). The XBB group exhibited significantly higher mean values of albumin (ALB), lymphocyte (LY), LY%, and eosinophilic cell (EO) compared to the BA.5.2 group (p<0.05), which is consistent with previous studies that identified decreased LY, LY%, EO, and ALB as biological predictors of poor prognosis.7–9 In contrast, the BA.5.2 group had significantly higher mean values of leucocyte (WBC), neutrophil (NEUT), NEUT%, C-reactive protein (CRP), aspartate aminotransferase (AST), and lactate dehydrogenase (LDH) than the XBB group (p<0.05), which is consistent with previous studies that identified elevated CRP, WBC, NEUT, NEUT%, and AST as biological predictors of poor prognosis.10 Furthermore, the length of hospitalization was significantly longer for the BA.5.2 group than for the XBB group (27.21±32.59 versus 13.70±16.85, p=0.001). However, there was no statistically significant difference in the extent of lung involvement between the two groups. This suggested that despite a higher proportion of patients with underlying diseases among XBB, they experience a shorter recovery time compared to BA.5.2 patients within the same pneumonia-affected area.
Comparison Between XBB and BA.5.2 Patients.
| Characteristics | XBB (n=66) | BA.5.2 (n=53) | p-Value |
|---|---|---|---|
| Age categories (yr) | |||
| <60 | 29 (43.9) | 17 (32.1) | 0.187 |
| ≥60 | 37 (56.1) | 36 (67.9) | |
| Gender | |||
| Male | 48 (72.7) | 34 (64.2) | 0.315 |
| Female | 18 (27.3) | 19 (35.8) | |
| Tobacco smoking | |||
| Current/former | 26 (39.4) | 6 (11.3) | 0.001 |
| Never | 40 (60.6) | 47 (88.7) | |
| Alcohol | |||
| Current/former | 12 (18.2) | 6 (11.3) | 0.299 |
| Never | 54 (81.8) | 47 (88.7) | |
| Diabetes | |||
| Yes | 12 (18.2) | 13 (25.0) | 0.368 |
| No | 54 (81.8) | 39 (75.0) | |
| Hypertension | |||
| Yes | 32 (48.5) | 34 (65.4) | 0.066 |
| No | 34 (51.5) | 18 (34.6) | |
| Cancer | |||
| Yes | 26 (39.4) | 9 (17.3) | 0.009 |
| No | 40 (60.6) | 43 (82.7) | |
| Severe COVID-19 | |||
| Yes | 20 (30.3) | 32 (60.4) | 0.001 |
| No | 46 (69.7) | 21 (39.6) | |
| Hospitalization day | 13.70±16.85 | 27.21±32.59 | 0.001 |
| Lung involvement | |||
| <50% | 44 (66.7) | 30 (56.6) | 0.261 |
| ≥50% | 22 (33.3) | 23 (43.4) | |
| Death | |||
| Yes | 9 (13.6) | 27 (50.9) | 0.001 |
| No | 57 (86.4) | 26 (49.1) | |
| Transplantation | |||
| Yes | 8 (12.1) | 10 (18.9) | 0.307 |
| No | 58 (87.9) | 43 (81.1) | |
| WBC | 7.98±5.07 | 9.71±5.88 | 0.029 |
| RBC | 3.88±0.81 | 3.71±0.73 | 0.223 |
| PLT | 187.3±102.2 | 183.3±78.3 | 0.742 |
| Hb | 109.9±25.9 | 108.7±21.6 | 0.681 |
| PCT | 0.70±1.89 | 0.17±0.07 | 0.138 |
| NEUT | 6.01±4.41 | 8.61±5.68 | 0.004 |
| NEUT% | 0.74±0.18 | 0.84±0.11 | 0.001 |
| EO | 0.10±0.15 | 0.05±0.12 | 0.012 |
| MO% | 0.07±0.04 | 0.05±0.03 | 0.076 |
| LY | 0.89±0.57 | 0.70±0.53 | 0.018 |
| LY% | 0.16±0.15 | 0.09±0.90 | 0.003 |
| FIB | 4.49±1.71 | 4.89±2.01 | 0.259 |
| D-dimer | 3.15±7.70 | 11.55±32.13 | 0.563 |
| CRP | 62.97±80.30 | 97.03±93.27 | 0.013 |
| ALB | 34.88±7.18 | 30.69±6.82 | 0.002 |
| AST | 48.20±59.15 | 82.74±94.93 | 0.004 |
| ALT | 32.89±40.01 | 42.09±37.27 | 0.071 |
| LDH | 329.8±292.9 | 407.1±214.0 | 0.005 |
| GGT | 74.94±92.54 | 86.86±185.56 | 0.977 |
Data are presented as number (percentage) or mean±SD. The bold of p-value means statistical significance.
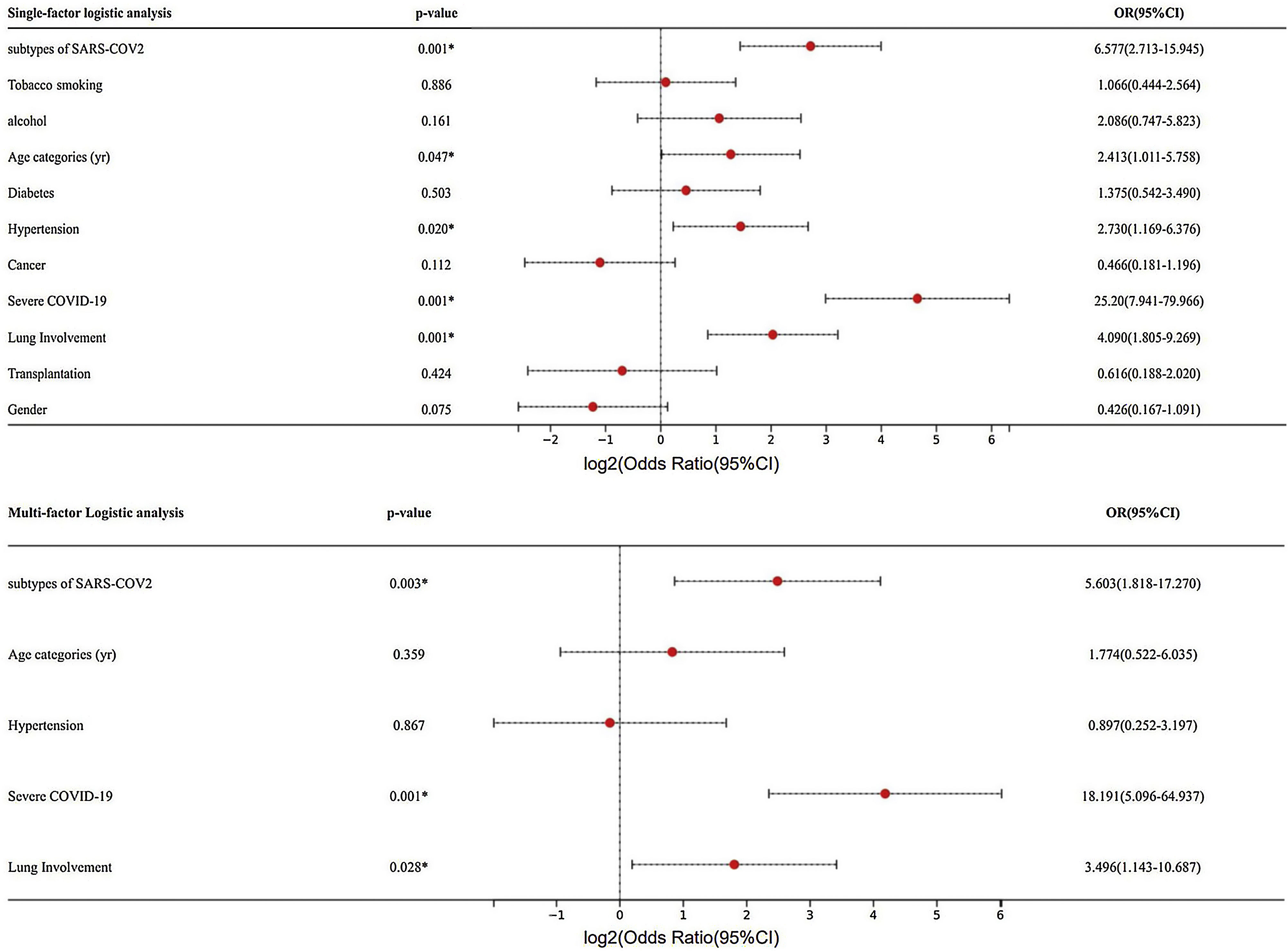
Further univariate logistic analysis of mortality revealed that subtypes of SARS-COV2 (p=0.030, OR=5.063, 95% CI: 2.713–15.945), age (p=0.047, OR=2.413, 95% CI: 1.011–5.758), hypertension (p=0.020, OR=2.730, 95% CI: 1.169–6.376), severe COVID-19 (p=0.001, OR=25.20, 95% CI: 7.941–79.966), and the extent of lung involvement (p=0.001, OR=4.090, 95% CI: 1.805–9.269) were significantly associated with mortality (Fig. 1). Subsequent multifactorial logistic regression analysis showed that mortality was significantly associated with subtypes of SARS-COV2 (p=0.003, OR=5.603, 95% CI: 1.818–17.270), severe COVID-19 (p=0.001, OR=18.191, 95% CI: 5.096–64.937), and the extent of lung involvement (p=0.028, OR=3.496, 95% CI: 1.143–10.687). After adjusting for multiple factors, including subtypes of SARS-COV2, hypertension, age, severe COVID-19, and lung area involved, the mortality rate among BA.5.2 patients remained higher than that of XBB patients, indicating that BA.5.2 is significantly more virulent than XBB, and therefore leading to a higher incidence of severe illness and mortality.
In conclusion, our study found that, BA.5.2 exhibits greater virulence, resulting in higher severity and mortality rates. These findings have significant implications for the treatment and management of patients with XBB and BA.5.2, highlighting the crucial role played by strain variation in determining the severity and outcome of COVID-19 cases, particularly during the ongoing pandemic.
FundingThis work was supported by the Natural Science Foundation of Guangdong Province (2021A1515010480 to Y.B. Zhou).
Conflicts of InterestNone declared.