Probe-based confocal laser endomicroscopy (pCLE) provides real-time vision of respiratory tissues at the cellular level through a flexible bronchoscope. This technique might guide sampling of pulmonary nodules,1–4 lymph nodes5 or pleural biopsies.6 First studies of the respiratory tract were performed with a 488nm wavelength probe that allows elastin visualization without adding fluorophores in the tissue. Because cells are not visualized, lung cancer pattern descriptions were limited to changes on the stromal component.1,7–9 Later studies used fluorophores like methylene blue or fluorescein that could be excited at a 488 or a 660nm wavelength, respectively, to visualize cell nuclei.2,4,10 In these studies, different imaging patterns were described. In particular, healthy tissue was described as having homogeneous architecture with bright, partially overlapping nuclei. Inflammation was considered when heterogeneous tissue architecture without overlapping nuclei but expanded cytoplasms were observed, and neoplasia was reported as a chaotic distribution of dark cells with heterogeneous nuclei.2,8,9,11,12 Although pictures of these patterns were frequently presented together with pictures of histology samples, none of the studies correlated the measurements performed in pCLE images to those of histology samples. In this pilot study, we aimed to explore the feasibility of correlating pCLE and optical microscopy features of normal and pathological airway samples.
Under general anesthesia and orotracheal intubation with a rigid bronchoscope (Efer-Dumon type, La Ciotat, France) pCLE was performed after applying a drop of 1% MB in nine regions of normal mucosa and in six tumors, as determined with white-light bronchoscopy. The AlveoFlex® probe and a laser scanning unit equipped with 660nm laser wavelength (Cellvizio®, Mauna Kea Technologies, Paris, France) were used. After pCLE image registration, biopsies were taken and histology was studied with optical microscopy following haematoxylin-eosin (H&E) staining. A total of 15 patients were studied. The biopsies revealed normal epithelium in 6 cases, inflammatory infiltrate in 3 cases and thoracic tumors in 6 cases, which included B cell lymphoma, adenocarcinoma, squamous cell carcinoma, small cell lung cancer, and non-small cell lung cancer. The study was approved by the local ethics review board (Clinical Research Ethics Committee of Bellvitge University Hospital – Act 08/13) and written informed consent was obtained from all participants.
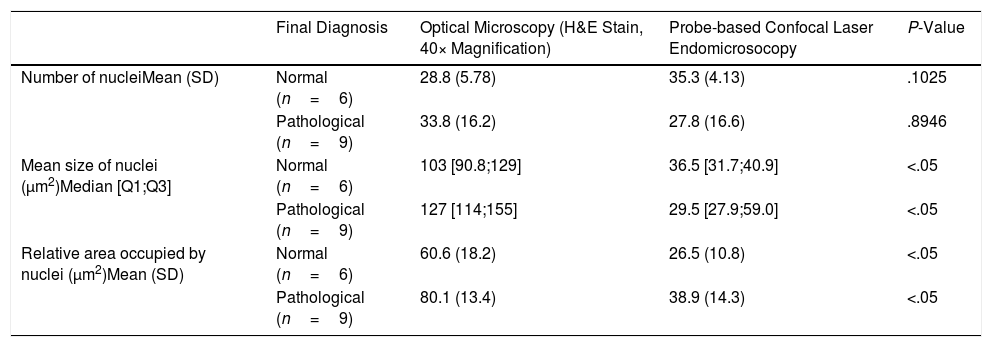
One representative image from the pCLE registration was selected and compared to one image of the H&E-stained sample. Nuclei were segmented in all images using ImageJ software (National Institutes of Health, Bethesda, MD, USA).13 In the pCLE images, we accounted for the number and mean size of nuclei, the relative area occupied by nuclei and the intensity of fluorescence. In the H&E-stained histological sample images, we accounted for the number and mean size of nuclei and the relative area occupied by nuclei. We chose these features because these structures can be identified both in the optical and the pCLE images.
We made two different comparisons. First, we compared the patterns of pCLE alone. A logistic regression model was fitted to predict the probability of pathological tissue (inflammation or malignancy) based on every feature. We observed that changes in these features were associated with variations in the probability of disease. In particular, we observed that the odds of disease increased by a factor of 1.03 (95% CI: 0.99–1.07) for every one unit increase in the mean size of nuclei, by a factor of 1 (95% CI: 1–1) for every one unit increase in the intensity of fluorescence, and by a factor of 1.088 (95% CI: 1.01–1.224) for every one unit increase in the relative area occupied by nuclei. Next, we registered the measurements performed in the optical microscopy and pCLE images (see Table 1) and compared their distributions. After a Wilcoxon rank sum exact test we observed that the distributions of the mean size and relative area occupied by nuclei were different in the images of optical compared to pCLE microscopy. Although this small analysis is not adequately powered to achieve statistical significance, our results of the pCLE alone patterns are in line with the previously mentioned studies showing that it is possible to discern between normal and pathological tissue patterns, while the measurements performed in the optical microscopy and pCLE images cannot be correlated to the tissue structures observed in the biopsy specimens.
Measurements Performed in the Histological Samples (Optical Microscopy) and pCLE Images.
| Final Diagnosis | Optical Microscopy (H&E Stain, 40× Magnification) | Probe-based Confocal Laser Endomicrosocopy | P-Value | |
|---|---|---|---|---|
| Number of nucleiMean (SD) | Normal (n=6) | 28.8 (5.78) | 35.3 (4.13) | .1025 |
| Pathological (n=9) | 33.8 (16.2) | 27.8 (16.6) | .8946 | |
| Mean size of nuclei (μm2)Median [Q1;Q3] | Normal (n=6) | 103 [90.8;129] | 36.5 [31.7;40.9] | <.05 |
| Pathological (n=9) | 127 [114;155] | 29.5 [27.9;59.0] | <.05 | |
| Relative area occupied by nuclei (μm2)Mean (SD) | Normal (n=6) | 60.6 (18.2) | 26.5 (10.8) | <.05 |
| Pathological (n=9) | 80.1 (13.4) | 38.9 (14.3) | <.05 |
Pathological refers to inflammation or malignancy.
H&E: haematoxylin–eosin, SD: standard deviation.
Our pilot study supports the use of pCLE to identify patterns of normal and pathological airway tissue but discourages comparisons between pCLE and histology images. These findings might reflect that the final histology diagnosis is based on a set of information not obtainable from a sequence of nuclei as it is observed in pCLE images. To empower pCLE as a useful tool for diagnosis of lung cancer, future studies should focus on identifying further discriminative features14 or specific tumor markers. Otherwise, this technique will be limited to the identification of pathological areas for biopsy guidance.
Authors’ ContributionsM.D-F, B.T. and A.R. generated the hypothesis; M.D-F., B.T. and N.B. designed the study; M.D-F., B.T, N.B, N.C, R.L., J.D and A.R contributed to data acquisition. C.T. performed the statistical analysis, and M.D-F. wrote the manuscript. All members of the study critically reviewed the submitted article for important intellectual content, provided final approval of the version to be published, and agreed to be accountable for all aspects of the work and to ensure that any questions related to the accuracy or integrity of any part of the work will be appropriately investigated and resolved.
FundingThis work was supported by grants from Fundació La Marató de TV3, SEPAR 2018, FUCAP-Albert Agustí and SOCAP.
Conflict of InterestAll authors of this manuscript report that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.