Cardiovascular diseases, lung cancer, diabetes mellitus, osteoporosis, anxiety, and depression are among the most prevalent comorbidities in patients with chronic obstructive pulmonary disease (COPD)1,2 and have a negative impact in both their quality of life as well as their survival.3,4 Divo et al.4 developed the COTE (COPD Specific Comorbidity Test) index after evaluating 1664 patients with COPD. Cancers of the lung, esophagus, breast, and pancreas, liver cirrhosis, atrial fibrillation or flutter, coronary heart disease, diabetes-associated neuropathy, pulmonary fibrosis, congestive heart failure, gastroduodenal ulcer, and anxiety were the pathologies with the greatest impact on the vital prognosis. Based on these findings, the group designed a bubble chart (mimicking the solar system) of the prevalence of these pathologies and their association with mortality and named it a “comorbidome”. However, distributions within this chart seem to be alterable and depend on the study population (in hospital vs outpatients).4,5 Therefore, we wondered whether the distribution of the comorbidome described by Divo et al. could vary even among different outpatient populations with COPD.
Our group has recently outlined that comorbidities, particularly the cardiovascular and psychiatric ones, impact the quality of life of patients with COPD (measured through the COMCOLD index), especially in the more symptomatic sub-groups according to the GOLD (Global Initiative for Chronic Obstructive Lung Disease) 2017 classification.6 In the present work, we performed a sub-analysis of the same population, aimed at reproducing the comorbidome depicted by Divo et al.,4 and evaluated (1) the impact of a panel of comorbidities on the survival of our outpatients with COPD and (2) their distribution within that comorbidome chart.
Briefly, retrospective observational study was performed on follow-up outpatients with COPD. Inclusion criteria were: age ≥40 years, current or former smoker with a pack-year index (PYI) ≥10 and a forced expiratory volume in the first second (FEV1)/forced vital capacity (FVC) ratio <0.70 past salbutamol administration. Exclusion criteria were chronic airflow obstruction and a PYI<10 or any pathology different from COPD. Patients were stratified according to the GOLD 2017 document. Information on the following comorbidities, included in the Charlson comorbidity index, was collected: myocardial infarction, angina, cardiovascular disease, cerebrovascular disease, dementia, COPD, connective tissue disease, gastrointestinal disease, mild or severe liver disease, complicated or uncomplicated diabetes mellitus, stroke, kidney failure, cancer, leukemia, lymphoma, secondary metastasis, and AIDS. Additionally evaluated pathologies were: arterial hypertension, dyslipidemia, cardiac arrhythmia, peripheral arterial disease, asthma, anxiety, and depression.
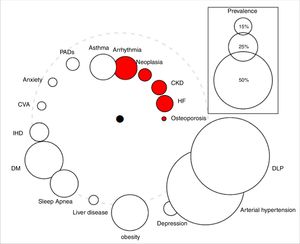
Qualitative variables were expressed as absolute values and percentages, while quantitative variables were summarized as means±SD. Cox logistic regression and Kaplan–Meier statistics were applied for time-dependent variables. The study was approved by the Clinical Research Ethics Committee of the University Hospital Nuestra Señora de la Candelaria (Spain). The comorbidome was plotted according to the original design of Divo et al.,4 where the circle diameter expressed the prevalence of the distinct comorbidities in percent and the distance of the circles to the center the risk of death based on the numerical value of the hazard ratio. The comorbidome represents comorbidities with a prevalence of more than 5% in the entire cohort.
A total of 439 patients were included in the study. The cohort characteristics had been described in a previous work.6 The patients’ mean age was 70; most of them were males with a predicted FEV1% of 55±20; 35% of them were current smokers. According to the GOLD 2017 categories, 142 (32%) were classified as GOLD A, 207 (48%) as GOLD B, 10 (2%) as GOLD C, and 80 (18%) as GOLD D. The mean Charlson index was 2.5±1.5.
During the follow-up period of 53 months, 131 patients (30%) died. Surviving patients were followed up for at least 41 months and a maximum of 88.5 months. The prevalence of comorbidities in both survivors and deceased patients is shown in Table 1. The following pathologies had the greatest impact on the vital prognosis of our patients: cardiac arrhythmia, chronic kidney disease (CKD), heart failure (HF), osteoporosis, and cancer (Table 1, Fig. 1).
Comorbidities of COPD and their association with risk of death.
| Comorbidities | Prevalence in non-deceased patients (n=308) | Prevalence in deceased patients (n=131) | Hazard ratioa (95% confidence interval) | P | |
|---|---|---|---|---|---|
| Osteoporosis, n (%) | 7 (2.3) | 9 (6.9) | 2.00 | 1.02–3.95 | .045 |
| HF, n (%) | 30 (9.7) | 33 (25.2) | 1.84 | 1.23–2.75 | .003 |
| CKD, n (%) | 28 (9.1) | 28 (21.5) | 1.66 | 1.08–2.56 | .020 |
| Neoplasia, n (%) | 24 (7.8) | 24 (18.3) | 1.57 | 1.00–2.44 | .049 |
| Arrhythmia, n (%) | 48 (15.6) | 41 (31.3) | 1.51 | 1.03–2.21 | .033 |
| Asthma, n (%) | 66 (21.4) | 33 (25.2) | 1.43 | 0.96–2.13 | .078 |
| Peripheral arterial disease, n (%) | 31 (10.1) | 18 (13.7) | 1.12 | 0.68–1.85 | .650 |
| Anxiety, n (%) | 24 (7.8) | 9 (6.9) | 1.11 | 0.56–2.19 | .763 |
| CVA, n (%) | 21 (6.8) | 11 (8.4) | 1.10 | 0.59–2.05 | .757 |
| IHD, n (%) | 45 (14.6) | 26 (19.8) | 1.07 | 0.70–1.65 | .757 |
| Diabetes mellitus, n (%) | 93 (30.2) | 50 (38.2) | 0.98 | 0.69–1.41 | .926 |
| SA, n (%) | 72 (23.4) | 33 (25.2) | 0.95 | 0.64–1.41 | .798 |
| Liver disease, n (%) | 27 (8.8) | 9 (6.9) | 0.91 | 0.46–1.80 | .792 |
| Obesity, n (%) | 97 (31.5) | 41 (31.3) | 0.82 | 0.56–1.11 | .285 |
| Depression, n (%) | 42 (13.6) | 13 (9.9) | 0.77 | 0.43–1.37 | .372 |
| Arterial hypertension, n (%) | 110 (35.7) | 39 (29.8) | 0.76 | 0.51–1.12 | .170 |
| Dyslipidemia, n (%) | 99 (32.1) | 45 (34.4) | 0.74 | 0.52–1.07 | .112 |
Abbreviations: HF: heart failure. CKD: chronic kidney disease. IHD: ischemic heart disease. CVA: cerebrovascular accidents. SA: sleep apnea.
The “comorbidome” is a graphic expression of comorbities with more than 5% prevalence in the entire cohort. The area of the circles relates to the prevalence of the disease. The proximity to the center (mortality) express the strength of the association between the disease and the risk of death. This was scaled from the inverse of the HR (1/HR). The dotted line represents HR=1. Beyond the line, HR are less than 1. The red bubble represents statistical significance association (HR>1; P<.05). Abbreviations: HF: heart failure. CKD: chronic kidney disease. IHD: ischemic heart disease. CVA: cerebrovascular accidents. DM: diabetes mellitus. DLP: dyslipidemia. PADs: peripheral arterial disease.
As shown in Fig. 1/Table 1, the impact of comorbidities on the survival of our outpatients diverges from that published by Divo et al.,4 thus reflecting differences between both comorbidomes. In contrast to the BODE cohort, CKD and osteoporosis did show a significant relationship with mortality. We need to stress that the latter, together with HF, had the greatest impact on survival. In our cohort, ischemic heart disease and neuropsychiatric pathologies were not related to the vital prognosis of the patients.
A number of works describe the negative impact of HF and CKD on COPD patients’ survival.4,7 The prevalence is even higher in overweight and obese subjects,8,9 and these account for up to 72% of our cohort. A study published by Verberne et al.8 shows that, in contrast to subjects with normal weight, obese COPD patients have an increased risk of diabetes (OR 3.79, CI95% 3.04–4.71), hypertension (OR 2.46, CI95% 2.07–2.93), and HF (OR 2.32, CI95% 1.55–3.46). The authors also detected significant inverse associations between patients’ survival and anxiety disorders (OR 0.49, CI 95% 0.28–0.86) and osteoporosis (OR 0.51, CI 95% 0.37–0.71). The latter comorbidity is classically described for patients with an emphysema phenotype, usually characterized by low weight, considerable air trapping, and a worse vital prognosis than the other phenotypes.10
We may not ignore the limitations of our study, i.e., the sample size, methodology—this is an observational study—and the fact that some of the comorbidities in the article by Divo et al.4 were not analyzed in our cohort. In contrast to the BODE cohort, patients with relevant comorbidities that prevented them from performing the 6-minute walk test were not excluded from our study. In addition, minimum follow-up was slightly longer in our study. In this sense, our work may better represent the patients with COPD that we find in healthcare practice. This prompted us to reflect on a general applicability of the COTE index (COPD specific comorbidity test) based on the comorbidome of Divo et al.,4 thinking that it should be substantiated in different patient populations. In summary, our study suggests that distributions within the comorbidome may vary according to the analyzed outpatient population.