Several studies have previously demonstrated that long-term exposure to outdoor pollution present airway inflammation in term of an increase of sputum neutrophils.
Aim and methodsThe aim of our study was to evaluate the level of airway inflammation by induced sputum in a group of 15 non-professionally exposed population of well-characterized COPD patients, residing in urban areas with high rate of outdoor pollution, compared to a control group of 13 individuals with COPD, living in rural areas with a low pollution rate. All participants underwent spirometry and sputum induction.
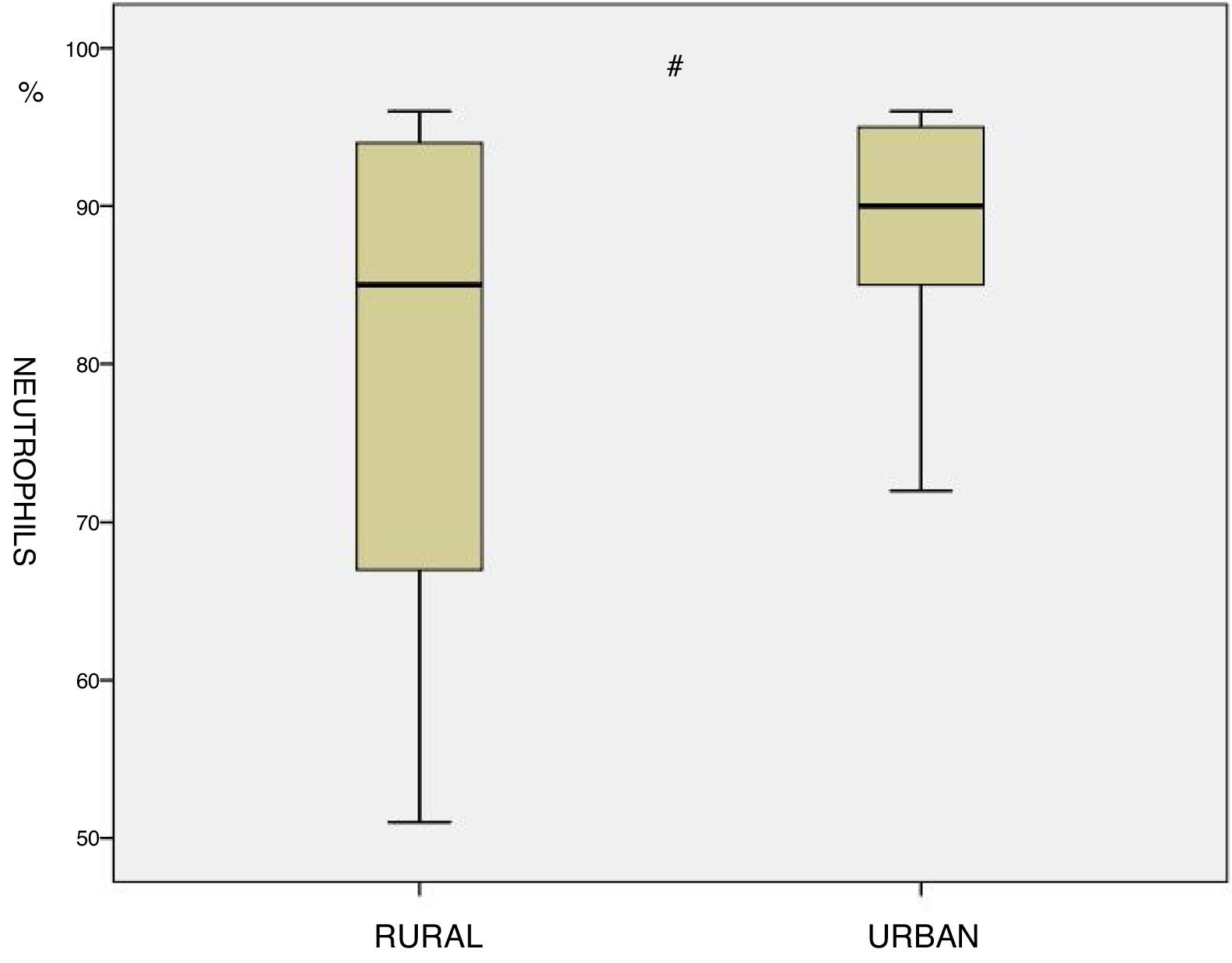
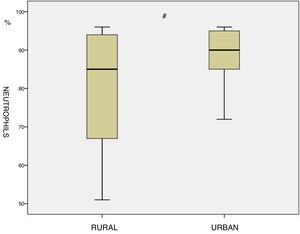
ResultsA statistically significant increase in the percentage of neutrophil cell count was found among the residents in urban areas compared to those living in rural regions (89.1 vs 79.0, p<0.05)
ConclusionsIn conclusion, we showed that non-professionally exposed patients with COPD residing in highly-polluted urban areas had greater airway inflammation in terms of sputum neutrophils compared to a population with very similar characteristics, living in rural areas with lower outdoor pollution. The results of this pilot study may be relevant for the long term effect of environmental outdoor pollution in vulnerable patients like those with COPD.
Varios estudios han demostrado con anterioridad que la exposición a la contaminación atmosférica a largo plazo provoca una inflamación de la vía aérea que se ve reflejada en un aumento de neutrófilos en el esputo.
Objetivo y métodosEl objetivo de nuestro estudio fue estimar el grado de inflamación de la vía aérea a través del esputo inducido en un grupo de 15 pacientes con EPOC bien caracterizada sin exposición por motivos profesionales y residentes en áreas urbanas con un nivel alto de contaminación atmosférica, comparados con un grupo control de 13 individuos con EPOC que vivían en zonas rurales con un nivel de contaminación atmosférica bajo. A todos los pacientes se les sometió a una espirometría y a una inducción del esputo.
ResultadosSe encontró un aumento estadísticamente significativo en el porcentaje de recuento celular de neutrófilos de los residentes en áreas urbanas en comparación con aquellos que vivían en zonas rurales (89,1 frente a 79,0%, p<0,05).
ConclusionesEn conclusión, demostramos que los pacientes con EPOC que no estaban expuestos debido a su profesión y residentes en áreas urbanas con alta contaminación atmosférica presentaban mayor inflamación de la vía aérea en cuanto al número de neutrófilos en esputo en comparación con una población de características muy similares residente en zonas rurales con menos contaminación atmosférica. El resultado de este estudio piloto podría ser relevante para el efecto a largo plazo de la contaminación atmosférica exterior en pacientes especialmente vulnerables como pueden ser aquellos con EPOC.
Chronic Obstructive Pulmonary Disease (COPD) represents one the most relevant causes of morbidity and mortality in both industrialized and developing countries.1
According to World Health Organization, COPD will very soon become the third cause of mortality throughout the world.2 During the last decades, a large number of epidemiological studies have provided evidence that outdoor air pollution is a contributing cause of morbidity and mortality in COPD.3
Moreover, ambient air pollutants such as particulate matter, carbon monoxide and nitrogen dioxide may be more harmful among subjects with pre-existing obstructive diseases (i.e. asthma, COPD).4
Over the last 25 years, induced sputum cellularity analysis has become a safe, robust and validated method to assess airways inflammation in asthma and COPD for both research and clinical purposes.5
It has previously demonstrated that groups of workers with long term exposure to air pollution present airways inflammation by an increase of sputum neutrophils.6,7
It would be interesting to extend the above observations by evaluating the inflammatory profile in a population of COPD patients without a professional exposure to pollutants but residing in an area with high rates of air pollution.
Therefore, the aim of our study was to evaluate the level of airways inflammation by induced sputum in a non-professionally exposed population of COPD patients, residing in urban areas with high rate of outdoor pollution, compared to a control group of individuals with COPD, living in rural areas with a low pollution rate.
Materials and methodsStudy populationA total number of 28 ex-smokers subjects (age range 50–80 years) participated to the study from January to December 2016. Participants were divided into 2 groups.
The first group was composed by 15 subjects with COPD residing for at least 30 years in a urban area with a high rate of outdoor pollution (city of Bari and Foggia, Apulia, Italy) without any professional exposure to pollutants.
The second group consisted of 13 individuals with COPD, residing from at least 30 years in a rural area with low rate of outdoor pollution (Daunian mountains, Apulia, Italy) without any professional exposure to pollutants.
All subjects were recruited from the outpatient clinics of the Respiratory Diseases Unit of University Hospital D’avanzo, Foggia, Italy and Respiratory Diseases Unit of University Hospital Policlinico, Bari, Italy.
COPD was defined according to the most recent guidelines.1 In short, the inclusion criteria were: history of smoking (current or ex) chronic symptoms of sputum production or dyspnea during efforts, post-bronchodilator FEV1/FVC ratio <70% and absence of clinical asthma or other pulmonary and cardiovascular abnormalities. None of them had experienced any exacerbations requiring corticosteroids and/or antibiotics in the previous 8 weeks. To avoid possible interference, also patients who had used inhaled corticosteroids in the last month were excluded from the study, whereas regular use of bronchodilators was allowed.
The present study was conducted according to the declaration of Helsinki, it was approved by a local ethical committee and all subjects signed an informed consent.
Study designAll participants had two separated visits with an interval of one week.
On visit one, detailed clinical anamnesis with smoke habits and occupational anamnesis were collected and clinical examination was performed.
On the second day spirometry, and induced sputum were performed.
Sputum inductionAfter baseline forced expiratory volume in the first second (FEV1) and forced vital capacity (FVC) measurements, salbutamol was administered by inhalation (400μg by MDI) and subjects inhaled hypertonic (4.5%) saline nebulized for periods of progressively increasing length (1, 2, 4, 8min). FEV1 was re-measured 1min after each inhalation period. An ultrasonic nebulizer (DeVilbiss 65, DeVilbiss Corporation, Somerset, PA, USA), was used to nebulize saline solutions.
Sputum processingSputum samples were analyzed within 2h from collection. Selected portions of the sputum sample originating from the lower respiratory tract were chosen using an inverted microscope and weighed. Dithiothreitol (DTT, Sputolysin, Calbiochem Corp, San Diego, CA, USA), freshly prepared in a dilution of one in 10 with distilled water, was added in a volume (in μl) equal to 4 times the weight of the sputum portion (in mg). Selected sputum was placed in a shaking water bath at 37°C for 20min and homogenized. Then, it was furtherly diluted with phosphate buffered saline in a volume equal to the sputum plus DTT. The suspension was subsequently filtered through gauze to remove mucus and was centrifuged at 1000rpm for 5min. The supernatant was aspirated and frozen at −70°C for later analysis. The cell pellet was resuspended in a volume of PBS equal to that of the sputum plus DTT and PBS as above. Total cell count (TCC) and viability (Trypan blue exclusion method) was determined using a Burkers chamber hemocytometer. The cell suspension was placed in a Shandon 3 cytocentrifuge (Shandon Southern Instruments, Sewickley, PA, USA) and cytospins will be prepared at 450rpm for 6min. Cytospin slides were fixed by methanol and were stained by May Grunwald Giemsa for an overall differential cell count on 500 nucleated non-squamous cells. Only samples with cell viability >50% and squamous cell contamination <20% were considered adequate.8
Statistical analysisBeing a “pilot study”, a sample size evaluation was not performed.
All statistical analyses were performed using SPSS software, version 16.0 (SPSS Inc., Chicago, USA). The results were expressed as mean and standard deviation for all factors. We verified normal data distribution and the comparison between groups was assessed by two tail t-student for all parameters.
A p-value <0.05 was considered statistically significant.
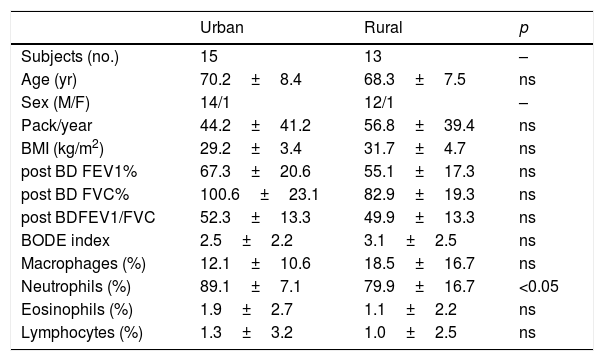
ResultsThe main subjects characteristics of both groups and induced sputum cell count are shown in Table 1. There are no significant differences in term of age, Body mass index (BMI), Pack-year index, BODE index and mean post bronchodilator FEV1, FVC and FEV1/FVC between the two groups. Residents in urban areas showed a significant increase in the percentage neutrophil cell count compared to inhabitants of rural areas (89.1 vs 79.0, p<0.05, Fig. 1). No other differences were identified with respect to the other cells analyzing even though in urban residents the number of macrophages was tendentially lower (12.1±10.6 vs 18.5±16.7, p=0.20).
Clinical characteristics and sputum cell count of the studied population. Values are expressed as mean±SD. BD=bronchodilator.
| Urban | Rural | p | |
|---|---|---|---|
| Subjects (no.) | 15 | 13 | – |
| Age (yr) | 70.2±8.4 | 68.3±7.5 | ns |
| Sex (M/F) | 14/1 | 12/1 | – |
| Pack/year | 44.2±41.2 | 56.8±39.4 | ns |
| BMI (kg/m2) | 29.2±3.4 | 31.7±4.7 | ns |
| post BD FEV1% | 67.3±20.6 | 55.1±17.3 | ns |
| post BD FVC% | 100.6±23.1 | 82.9±19.3 | ns |
| post BDFEV1/FVC | 52.3±13.3 | 49.9±13.3 | ns |
| BODE index | 2.5±2.2 | 3.1±2.5 | ns |
| Macrophages (%) | 12.1±10.6 | 18.5±16.7 | ns |
| Neutrophils (%) | 89.1±7.1 | 79.9±16.7 | <0.05 |
| Eosinophils (%) | 1.9±2.7 | 1.1±2.2 | ns |
| Lymphocytes (%) | 1.3±3.2 | 1.0±2.5 | ns |
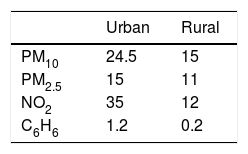
The mean yearly values of 2016 expressed as μg/m3 for particulate matter with a diameter between 2.5 and 10μm (PM10), fine particles with a diameter of 2.5μm or less (PM2.5), nitrogen dioxide (NO2) and benzene (C6H6) at both urban and rural sites are shown in Table 2. Measurements of pollutants come from the same urban and rural areas where subjects were recruited. By a pure data observation, level of pollutants appeared higher in urban areas compared to rural zones as expected (see Table 2).
DiscussionIn the current study we demonstrated that non-professionally exposed patients with COPD residing in highly-polluted urban areas had greater airway inflammation in terms of sputum neutrophils compared to a population with very similar characteristics, living in rural areas with lower outdoor pollution.
To the best of our knowledge, this is the first study that specifically investigated sputum cellularity in COPD patients belonging to two different classes of environmental air pollution exposure.
A link between air pollution and cytological changes in sputum has been known since the 1970s9 and more recently, Wallace et al.4 showed an association between proximity to high traffic roads and sputum neutrophils, irrespective of the physiological abnormality (i.e. asthma or COPD). Moreover, subjects with asthma with acute exposure to diesel pollution showed an increase in sputum neutrophils without modifications in eosinophils.10 As previously mentioned, a number of authors have formerly investigated the airway inflammation by induced sputum in workers exposed to outdoor pollution such as traffic policemen and waste handlers, showing an increase of neutrophils6,7 and IL-8.6 Our findings were in line with the above data.
The strengths of our study were the strict selection of COPD patients, using worldwide accepted guidelines1 and the reliability of our measurements of airway inflammation, made by a well-trained operator in a fully equipped and certified laboratory.
The limitations of the present study were the relatively small number of enrolled patients, without a sample size estimation. Undoubtedly, the data deriving from this pilot study require further investigations with much higher sample sizes. Furthermore, we did not perform direct measurements of outdoor pollutants in the residing areas of our population. However, we obtained data of airborne pollutants from officially verified measurements that are publicly available online (www.arpa.puglia.it/web.guest/qariainq). By a pure data observation, the level of PM10, PM2.5, NO2 and C6H6 appeared higher in urban areas compared to rural zones as predictable (see Table 2).
It may be argued that our data show a 15% difference in post-BD FEV1% and 17.7% post-BD FVC% between the two groups. Despite this (non-statistically significant) difference in lung volumes, the two groups of COPD patients had comparable clinical characteristics, as reflected by a similar BODE index. Nevertheless, it would be very interesting to follow these patients longitudinally for observing clinical and functional COPD progression between the two sites. Further investigations should also include direct measurement of airborne allergens in both urban and rural areas, as this may have an impact of respiratory symptoms and lung function.
In conclusion, considering our population of patients, the current data suggest that the neutrophilia, normally present in COPD as a feature of the disease, may have been modified by the effect of air pollutants. Our study also confirmed the importance of using induced sputum for assessing airway inflammation and to investigate the biological effects of air pollutants among the population. Warranting further studies, our findings may have relevant public health implications as environmental outdoor pollution can determine higher risks to respiratory health in vulnerable patients like those with COPD, thus promoting appropriate strategies of prevention.
Conflict of interestsThis study was independent and without external fundings. None of the authors have any conflict of interest to declare.