Although older adults represent a significant proportion of patients with venous thromboembolism (VTE), the data on the impact of age-related differences in the clinical presentation, management, and outcomes of VTE are scarce.
MethodsWe analyzed data from the RIETE registry database, an ongoing global observational registry of patients with objectively confirmed VTE, to compare patient characteristics, clinical presentation, treatments, and outcomes between elderly (≥70 years) vs. non-elderly (<70 years) patients.
ResultsFrom January 2001 to March 2021, 100,000 adult patients were enrolled in RIETE. Elderly patients (47.9%) were more frequently women (58.2% vs. 43.5%), more likely had unprovoked VTE (50.5% vs. 45.1%) and most often presented with severe renal failure (10.2% vs. 1.2%) and acute pulmonary embolism (PE) (vs. deep vein thrombosis) (54.3% vs. 44.5%) compared to non-elderly patients (p<0.001 for all comparisons). For the PE subgroup, elderly patients more frequently had non-low risk PE (78.9% vs. 50.7%; p<0.001), respiratory failure (33.9% vs. 21.8%; p<0.001) and myocardial injury (40.0% vs. 26.2%; p<0.001) compared to non-elderly patients. Thrombolysis (0.9% vs. 1.7%; p<0.001) and direct oral anticoagulants (8.8% vs. 11.8%; p<0.001) were less frequently administered to elderly patients. Elderly patients showed a significantly higher 30-day all-cause mortality (adjusted odds ratio [OR] 1.36, 95%CI: 1.22–1.52) and major bleeding (OR, 2.08; 95%CI, 1.85–2.33), but a lower risk of 30-day VTE recurrences (OR, 0.62, 95%CI, 0.54–0.71).
ConclusionsCompared with non-elderly patients, elderly patients had a different VTE clinical profile. Advanced therapies were less frequently used in older patients. Age was an independent predictor of mortality.
Venous thromboembolism (VTE), which includes deep vein thrombosis (DVT) and pulmonary embolism (PE), remains a worldwide major health problem. The annual incidence of VTE in the general population is estimated as around 1–2 cases per 1000 persons,1,2 which progressively increases with the patient's age.3–5 A significant proportion of patients with VTE are elderly,6–8 but our knowledge of the impact of age on the presentation, management, and outcomes of VTE is still limited.6,9,10 Older age has been related to worse outcomes in VTE, which can be partly explained by a higher comorbidity burden and a lower cardiopulmonary reserve.4,11 Defining age-related differences in the presentation and natural history of VTE is therefore critical to improving our comprehension of the disease's epidemiology and optimizing its management.12,13 Nevertheless, older patients are underrepresented in clinical trials and few data are available on the differential effect of pharmacological and interventional therapies for VTE in terms of age subgroups.13–15 Increasing morbidities, frailty, polypharmacy, and age-related changes in pharmacokinetics are crucial factors associated with elderly people in “real-world” clinical practice.16
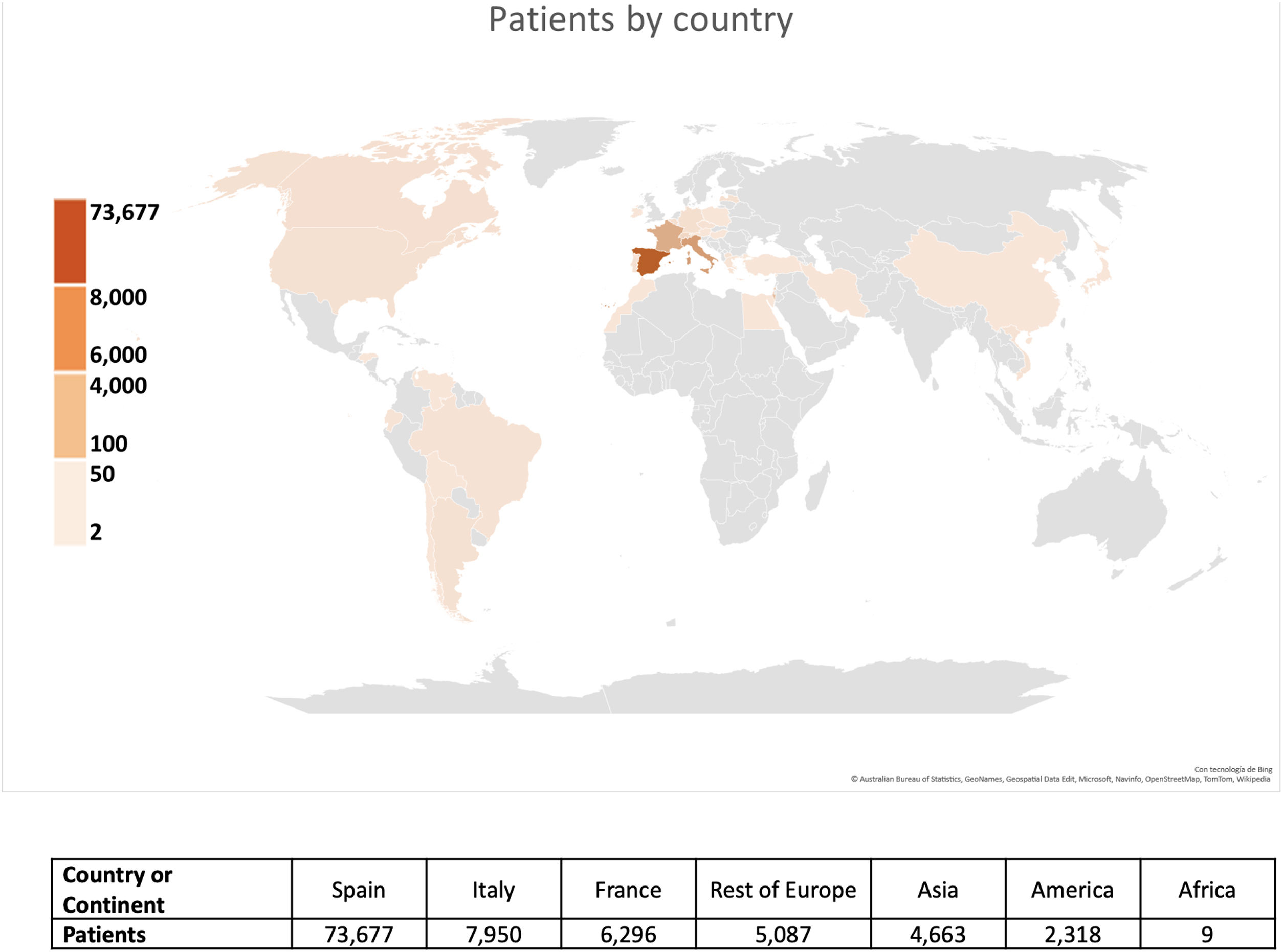
Clinical registries are an excellent source of information that enables us to address this critical gap in our knowledge. The RIETE (Registro Informatizado de Enfermedad TromboEmbólica) registry is the largest ongoing, observational, multicenter, and international registry of consecutive patients with objectively confirmed acute VTE.17 This registry, created in Spain in 2001, initially focused on DVT and PE in Spain, in order to explore the natural history of VTE in patients with special conditions who are usually excluded from randomized clinical trials, and learn how to prevent and treat this disease more effectively.18 The registry subsequently expanded to embrace several other countries and other forms of VTE, including superficial vein thrombosis, splanchnic vein thrombosis, retinal vein thrombosis, and cerebral vein thrombosis. The RIETE registry recently reached the milestone of 100,000 enrolled patients with confirmed acute VTE, collected from 421 collaborating centers distributed among 31 countries (Fig. 1).
Therefore, the aim of the current study was to compare the baseline clinical characteristics, initial VTE presentation, treatment strategies, and outcomes between elderly (≥70 years) and non-elderly (<70 years) patients in the first 100,000 patients with acute VTE enrolled in the RIETE registry.
MethodsStudy design and oversightWe conducted a study of prospectively recruited adult patients enrolled in RIETE who had objectively confirmed symptomatic or asymptomatic VTE. The rationale and methodology have been already reported elsewhere.17
Patient selectionThe patients were screened on each participating site by the site investigators and checked for their eligibility. Consecutive patients enrolled in the RIETE from January 1, 2001 onwards were included. Confirmatory testing included high-probability ventilation-perfusion scintigraphy,19 positive contrast-enhanced, PE-protocol, helical chest computed tomography (CT) for PE (single or multidetector CT),20 or venous compression ultrasonography positive for proximal and distal DVT.21 Patients were excluded if they were currently participating in a therapeutic clinical trial with a blinded therapy. Patients with distal DVT or thrombosis on unusual sites were not excluded, and nor were those with incidental (symptomatic or asymptomatic) VTE.
Variables and definitionsBaseline characteristics around the time of VTE diagnosis included demographics, coexisting or underlying conditions and therapies, risk factors for VTE, and the initial VTE presentation. The type, dose, and duration of anticoagulation therapy upon VTE diagnosis were also recorded.
Elderly patients were defined as those who were aged 70 years or more.7,22,23 Surgical patients were defined as those who had undergone major surgery in the 2 months prior to VTE. Immobilized patients were defined as non-surgical patients who had been recently assigned to bed rest with or without bathroom privileges for ≥4 days in the 2-month period prior to VTE diagnosis. Active cancer was defined as newly diagnosed cancer or the administration of anti-neoplastic treatment of any type (i.e., surgery, chemotherapy, radiotherapy, hormonal, support therapy, or combined therapies). High body weights were defined as 100kg or more. Unprovoked VTE was considered in the absence of active cancer, recent immobility, surgery, estrogen use, pregnancy, postpartum, or long-term travel. Anemia was defined as hemoglobin levels<13g/dL for men and <12g/dL for women.24 Asymptomatic VTE was considered in patients with no VTE symptoms who were subjected to imaging tests for other reasons. Creatinine clearance levels were measured using the Cockroft-Gault formula. Renal failure was defined as creatinine clearance<60mL/min, and as severe with a creatinine clearance<30mL/min. Patients initially presenting with PE were categorized according to the simplified Pulmonary Embolism Severity Index (sPESI) and identified as low-risk PE, when the sPESI was 0 points.25 Extension of pulmonary artery clots were defined as proximal when the filling defects were found in central, main and/or lobar pulmonary arteries, and peripheral when only occurring in segmental and/or subsegmental arterial branches.
Study outcomesThe primary outcome was 30-day all-cause mortality. Secondary outcomes included (1) PE-related mortality, which was defined, in the absence of autopsy, as any death appearing within 10 days of a symptomatic, objectively confirmed PE event; (2) symptomatic recurrent VTE, confirmed by a confirmatory imaging test; (3) major bleeding (bleeding occurring on a critical site [retroperitoneal, spinal, or intracranial], bleeding requiring transfusion of at least two units of blood, or fatal bleeding) within the 30-days of follow-up26; (4) clinically relevant non-major bleeding (CRNMB), when the bleeding required medical assistance but did not meet the criteria for major bleeding; and (5) arterial ischemic events (i.e., ischemic stroke, myocardial infarction or limb amputation). We also examined rates of all-cause and PE-related mortality, recurrent VTE and major bleeding within 90 days of the diagnosis of VTE.
Treatment and follow-upPatients were managed according to the current clinical practice of each participating hospital. Patients were followed up for a minimum of three months, although a longer follow-up was recommended, whenever possible. During this period, each episode of clinically suspected recurrent VTE was investigated by repeat imaging studies, as appropriate.
Statistical analysisThe present report adheres to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement.27 Summary statistics were calculated for all the patients’ variables. Variables with >2% and <5% of missing data were imputed. We imputed these variables’ missing data (e.g., isolated lower-limb DVT, isolated upper-extremity DVT) using the multiple imputations.28 We used unpaired two-tailed t-tests or the Mann–Whitney U test (for those variables found not to follow a normal distribution) for comparisons in the distributions of continuous variables between elderly or non-elderly patients, and chi-squared or Fisher's exact tests to compare categorical data between both groups.
Incidence rates of recurrent VTE, bleedings, and death were compared using the odds ratio (OR) and 95% confidence intervals (CI). The impact of age (i.e., elderly vs. non-elderly) on primary outcome was evaluated using univariable and multivariable logistic regression. For multivariable models, we considered factors previously demonstrated to be prognostically significant or thought to be clinically important, as well as covariates identified in bivariable analyses as predictors of mortality.
Statistical analysis was carried out using SPSS software, version 25 (SPSS, Inc., Chicago, IL, USA), and a two-sided p<0.05 was considered to be statistically significant.
ResultsAs of March 2021, RIETE reached 100,000 patients with confirmed VTE. Of these, 50,560 (50.6%) patients were women, and 47,884 (47.9%) were elderly patients. Overall, 51,849 (51.8%) presented with PE, 39,403 (39.4%) with isolated lower-limb DVT, 2169 (2.2%) with isolated upper-extremity DVT, 1848 (1.8%) with superficial vein thrombosis and 4007 (4.0%) with VTE on other sites (e.g., retinal, cerebral sinus). Overall, 3855 (3.9%) acute VTE patients were incidentally identified.
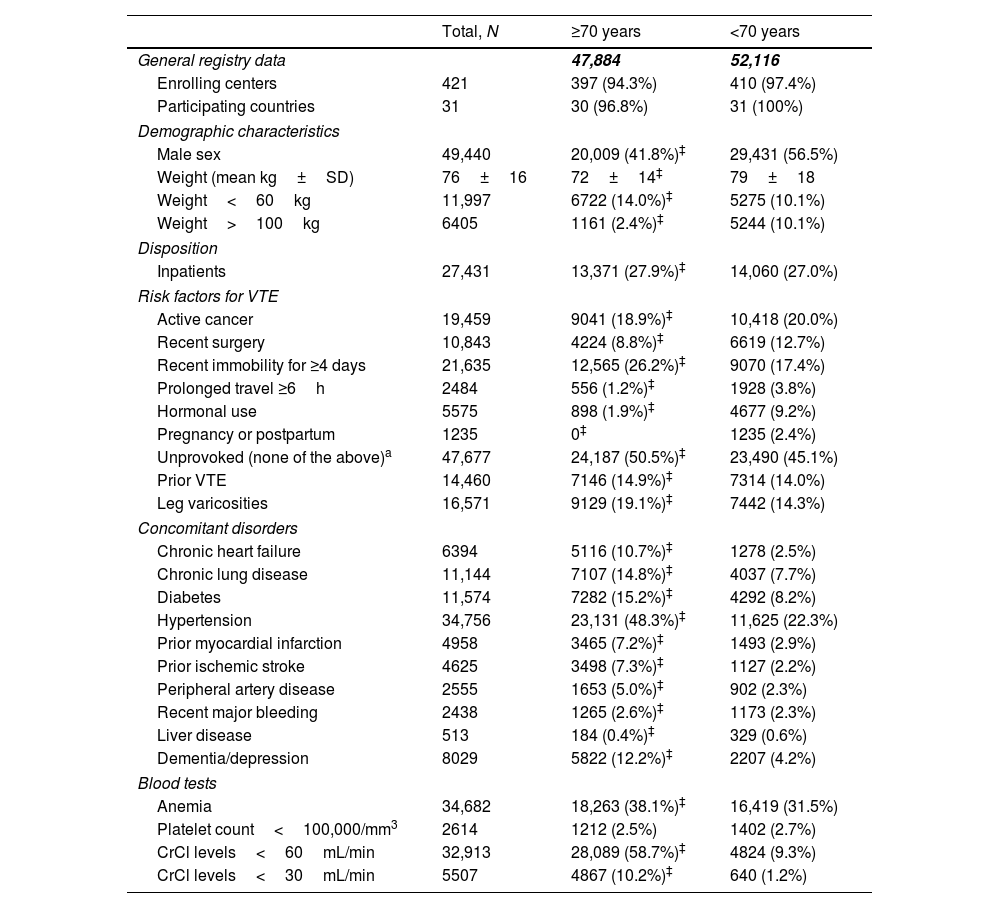
Clinical characteristicsMale gender (41.8% vs. 56.5%) and high body weights (2.4% vs. 10.1%) were less common in elderly patients than in non-elderly patients (p<0.001 for all comparisons). Compared with non-elderly patients, elderly patients were more likely to be immobilized (26.2% vs. 17.4%), and less likely to have recent surgery (8.8% vs. 13%) or a prolonged travel (1.2% vs. 3.8%) prior to the VTE episode (p<0.001 for all comparisons). Active cancer at baseline was observed in 18.9% and 20.0% of elderly and non-elderly patients, respectively. Moreover, elderly patients were more likely to have an unprovoked VTE (50.5% vs. 45.1%; p<0.001). Medical co-morbidities were more frequently observed in older patients, including chronic heart and lung disease (10.7% vs. 2.5%; and 14.8% vs. 7.7%, respectively), diabetes (15.2% vs. 8.2%), systemic hypertension (48.3% vs. 22.3%), and peripheral artery disease (5.0% vs. 2.3%) (p<0.001 for all comparisons). Overall, renal failure at baseline was observed in 58.7% and 9.3% of elderly and non-elderly patients, respectively, and severe renal dysfunction was observed in 10.2% of elderly patients (vs. 1.2% in non-elderly; p<0.001) (Table 1).
Clinical characteristics at baseline in the first 100,000 patients with acute VTE enrolled in the RIETE registry, according to age.
| Total, N | ≥70 years | <70 years | |
|---|---|---|---|
| General registry data | 47,884 | 52,116 | |
| Enrolling centers | 421 | 397 (94.3%) | 410 (97.4%) |
| Participating countries | 31 | 30 (96.8%) | 31 (100%) |
| Demographic characteristics | |||
| Male sex | 49,440 | 20,009 (41.8%)‡ | 29,431 (56.5%) |
| Weight (mean kg±SD) | 76±16 | 72±14‡ | 79±18 |
| Weight<60kg | 11,997 | 6722 (14.0%)‡ | 5275 (10.1%) |
| Weight>100kg | 6405 | 1161 (2.4%)‡ | 5244 (10.1%) |
| Disposition | |||
| Inpatients | 27,431 | 13,371 (27.9%)‡ | 14,060 (27.0%) |
| Risk factors for VTE | |||
| Active cancer | 19,459 | 9041 (18.9%)‡ | 10,418 (20.0%) |
| Recent surgery | 10,843 | 4224 (8.8%)‡ | 6619 (12.7%) |
| Recent immobility for ≥4 days | 21,635 | 12,565 (26.2%)‡ | 9070 (17.4%) |
| Prolonged travel ≥6h | 2484 | 556 (1.2%)‡ | 1928 (3.8%) |
| Hormonal use | 5575 | 898 (1.9%)‡ | 4677 (9.2%) |
| Pregnancy or postpartum | 1235 | 0‡ | 1235 (2.4%) |
| Unprovoked (none of the above)a | 47,677 | 24,187 (50.5%)‡ | 23,490 (45.1%) |
| Prior VTE | 14,460 | 7146 (14.9%)‡ | 7314 (14.0%) |
| Leg varicosities | 16,571 | 9129 (19.1%)‡ | 7442 (14.3%) |
| Concomitant disorders | |||
| Chronic heart failure | 6394 | 5116 (10.7%)‡ | 1278 (2.5%) |
| Chronic lung disease | 11,144 | 7107 (14.8%)‡ | 4037 (7.7%) |
| Diabetes | 11,574 | 7282 (15.2%)‡ | 4292 (8.2%) |
| Hypertension | 34,756 | 23,131 (48.3%)‡ | 11,625 (22.3%) |
| Prior myocardial infarction | 4958 | 3465 (7.2%)‡ | 1493 (2.9%) |
| Prior ischemic stroke | 4625 | 3498 (7.3%)‡ | 1127 (2.2%) |
| Peripheral artery disease | 2555 | 1653 (5.0%)‡ | 902 (2.3%) |
| Recent major bleeding | 2438 | 1265 (2.6%)‡ | 1173 (2.3%) |
| Liver disease | 513 | 184 (0.4%)‡ | 329 (0.6%) |
| Dementia/depression | 8029 | 5822 (12.2%)‡ | 2207 (4.2%) |
| Blood tests | |||
| Anemia | 34,682 | 18,263 (38.1%)‡ | 16,419 (31.5%) |
| Platelet count<100,000/mm3 | 2614 | 1212 (2.5%) | 1402 (2.7%) |
| CrCl levels<60mL/min | 32,913 | 28,089 (58.7%)‡ | 4824 (9.3%) |
| CrCl levels<30mL/min | 5507 | 4867 (10.2%)‡ | 640 (1.2%) |
Comparisons between patients<70 years and older than 70 years.
*p<0.05.
†p<0.01.
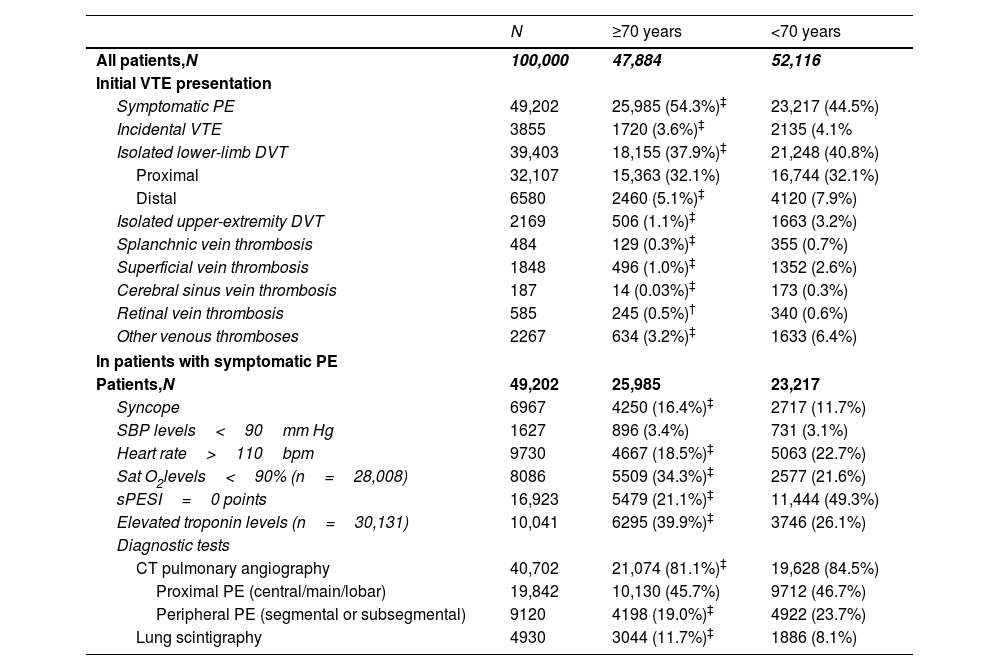
In comparison with non-elderly patients, acute symptomatic PE (54.3% vs. 44.5%; p<0.001) and non-low risk PE (78.9% vs. 50.7%; p<0.001) were more common in elderly patients, whereas incidental VTE was less common (3.6% vs. 4.1%; p<0.001) (Table 2). The mean length of hospital stay was higher in non-elderly patients (9.8 vs. 9.1 days, p<0.001) (Table 3).
Initial VTE presentation in the first 100,000 patients recruited in the RIETE registry, according to age.
| N | ≥70 years | <70 years | |
|---|---|---|---|
| All patients,N | 100,000 | 47,884 | 52,116 |
| Initial VTE presentation | |||
| Symptomatic PE | 49,202 | 25,985 (54.3%)‡ | 23,217 (44.5%) |
| Incidental VTE | 3855 | 1720 (3.6%)‡ | 2135 (4.1% |
| Isolated lower-limb DVT | 39,403 | 18,155 (37.9%)‡ | 21,248 (40.8%) |
| Proximal | 32,107 | 15,363 (32.1%) | 16,744 (32.1%) |
| Distal | 6580 | 2460 (5.1%)‡ | 4120 (7.9%) |
| Isolated upper-extremity DVT | 2169 | 506 (1.1%)‡ | 1663 (3.2%) |
| Splanchnic vein thrombosis | 484 | 129 (0.3%)‡ | 355 (0.7%) |
| Superficial vein thrombosis | 1848 | 496 (1.0%)‡ | 1352 (2.6%) |
| Cerebral sinus vein thrombosis | 187 | 14 (0.03%)‡ | 173 (0.3%) |
| Retinal vein thrombosis | 585 | 245 (0.5%)† | 340 (0.6%) |
| Other venous thromboses | 2267 | 634 (3.2%)‡ | 1633 (6.4%) |
| In patients with symptomatic PE | |||
| Patients,N | 49,202 | 25,985 | 23,217 |
| Syncope | 6967 | 4250 (16.4%)‡ | 2717 (11.7%) |
| SBP levels<90mm Hg | 1627 | 896 (3.4%) | 731 (3.1%) |
| Heart rate>110bpm | 9730 | 4667 (18.5%)‡ | 5063 (22.7%) |
| Sat O2levels<90% (n=28,008) | 8086 | 5509 (34.3%)‡ | 2577 (21.6%) |
| sPESI=0 points | 16,923 | 5479 (21.1%)‡ | 11,444 (49.3%) |
| Elevated troponin levels (n=30,131) | 10,041 | 6295 (39.9%)‡ | 3746 (26.1%) |
| Diagnostic tests | |||
| CT pulmonary angiography | 40,702 | 21,074 (81.1%)‡ | 19,628 (84.5%) |
| Proximal PE (central/main/lobar) | 19,842 | 10,130 (45.7%) | 9712 (46.7%) |
| Peripheral PE (segmental or subsegmental) | 9120 | 4198 (19.0%)‡ | 4922 (23.7%) |
| Lung scintigraphy | 4930 | 3044 (11.7%)‡ | 1886 (8.1%) |
Comparisons between patients<70 years and older than 70 years.
*p<0.05.
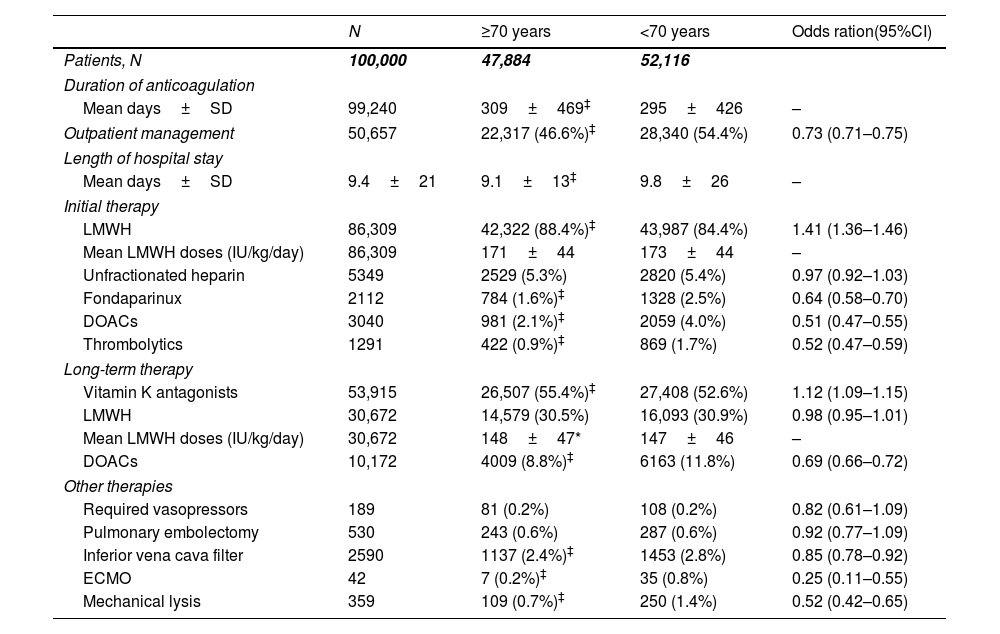
Treatment strategies for initial and long-term anticoagulant therapy in the first 100,000 patients in the RIETE registry, according to age.
| N | ≥70 years | <70 years | Odds ration(95%CI) | |
|---|---|---|---|---|
| Patients, N | 100,000 | 47,884 | 52,116 | |
| Duration of anticoagulation | ||||
| Mean days±SD | 99,240 | 309±469‡ | 295±426 | – |
| Outpatient management | 50,657 | 22,317 (46.6%)‡ | 28,340 (54.4%) | 0.73 (0.71–0.75) |
| Length of hospital stay | ||||
| Mean days±SD | 9.4±21 | 9.1±13‡ | 9.8±26 | – |
| Initial therapy | ||||
| LMWH | 86,309 | 42,322 (88.4%)‡ | 43,987 (84.4%) | 1.41 (1.36–1.46) |
| Mean LMWH doses (IU/kg/day) | 86,309 | 171±44 | 173±44 | – |
| Unfractionated heparin | 5349 | 2529 (5.3%) | 2820 (5.4%) | 0.97 (0.92–1.03) |
| Fondaparinux | 2112 | 784 (1.6%)‡ | 1328 (2.5%) | 0.64 (0.58–0.70) |
| DOACs | 3040 | 981 (2.1%)‡ | 2059 (4.0%) | 0.51 (0.47–0.55) |
| Thrombolytics | 1291 | 422 (0.9%)‡ | 869 (1.7%) | 0.52 (0.47–0.59) |
| Long-term therapy | ||||
| Vitamin K antagonists | 53,915 | 26,507 (55.4%)‡ | 27,408 (52.6%) | 1.12 (1.09–1.15) |
| LMWH | 30,672 | 14,579 (30.5%) | 16,093 (30.9%) | 0.98 (0.95–1.01) |
| Mean LMWH doses (IU/kg/day) | 30,672 | 148±47* | 147±46 | – |
| DOACs | 10,172 | 4009 (8.8%)‡ | 6163 (11.8%) | 0.69 (0.66–0.72) |
| Other therapies | ||||
| Required vasopressors | 189 | 81 (0.2%) | 108 (0.2%) | 0.82 (0.61–1.09) |
| Pulmonary embolectomy | 530 | 243 (0.6%) | 287 (0.6%) | 0.92 (0.77–1.09) |
| Inferior vena cava filter | 2590 | 1137 (2.4%)‡ | 1453 (2.8%) | 0.85 (0.78–0.92) |
| ECMO | 42 | 7 (0.2%)‡ | 35 (0.8%) | 0.25 (0.11–0.55) |
| Mechanical lysis | 359 | 109 (0.7%)‡ | 250 (1.4%) | 0.52 (0.42–0.65) |
Comparisons between patients<70 years and older than 70 years.
Elderly patients with PE were more likely to present with syncope (16.4% vs. 11.7%), oxygen saturation <90% (33.9% vs. 21.8%), and elevated troponin levels (40.0% vs. 26.2%) (p<0.001 for all comparisons). Nevertheless, a comparable proportion of patients had hemodynamically unstable PE (i.e., systolic blood pressure<90mmHg) at presentation (3.4% vs. 3.1%) (Table 2).
Pharmacological and reperfusion therapiesFor initial therapy, most patients in both subgroups received low-molecular-weight heparin (LMWH) as initial therapy (88.4% of elderly and 84.4% of non-elderly patients, respectively) but elderly patients were less likely to receive direct oral anticoagulants (DOAC) (2.1 vs. 4.0%, p<0.001) and fondaparinux (1.6% vs. 2.5%, p<0.001) than non-elderly patients.
As regards the use of advanced therapies and supportive measures, elderly patients received reperfusion treatment less frequently, including systemic thrombolysis (0.9% vs. 1.7%, p<0.001) and catheter-directed therapies (0.7% vs. 1.4%, p<0.001). Moreover, use of inferior vena cava (IVC) filter (2.4% vs. 2.8%, p<0.001) and extracorporeal membrane oxygenation (ECMO) (0.2% vs. 0.8%, p<0.001) were also less likely used in elderly patients. Vasopressor agents and surgical embolectomy were similarly offered to only a low proportion of both elderly and non-elderly VTE patients (0.2% and 0.6%, respectively).
As for long-term therapy, 52.6% of non-elderly patients and 55.4% of non-elderly patients received vitamin K antagonists (VKAs). Although the use of DOACs increased significantly over the course of the study, a small proportion of patients received DOACs (10.2%), less often in elderly patients, compared to non-elderly ones (8.8% vs. 11.8%, p<0.001). Of those patients receiving long-term LMWH (30.7%), a similar proportion of use and mean daily doses was found in both age groups (Table 3 and Fig. S1).
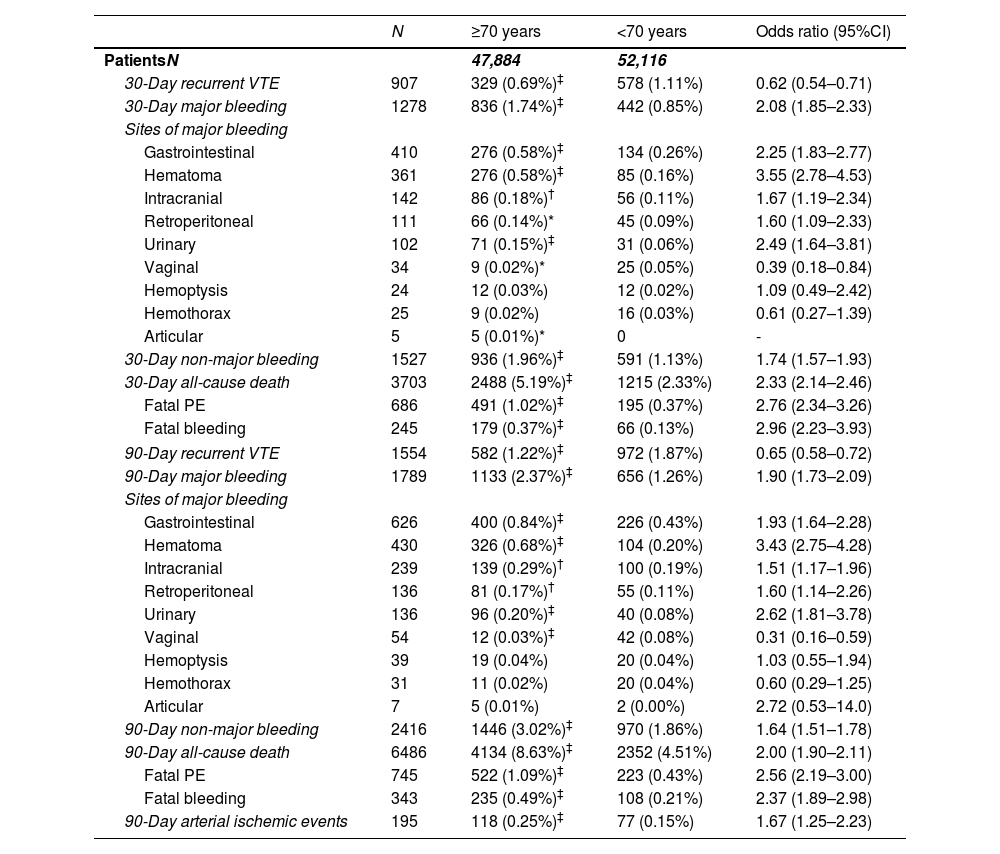
OutcomesThe entire cohort had a 30-day and 90-day all-cause mortality rate of 3.7% and 6.5%, respectively. Compared with non-elderly patients, elderly patients presented a higher 30-day all-cause (5.2% vs. 2.3%; OR 2.30, 95%CI: 2.14–2.46) and PE-related mortality (1.0% vs. 0.4%; OR 2.76, 95%CI: 2.34–3.26) in the univariable analysis. At 30 days, elderly patients had an increased risk of major bleeding (1.7% vs. 0.8%; OR 2.08, 95%CI 1.85–2.33), including gastrointestinal hemorrhages (0.6% vs. 0.3%, OR 2.25, 95%CI 1.83–2.77) and fatal bleedings (0.4% vs. 0.1%, OR 2.96, 95%CI 2.23–3.93). Furthermore, elderly patients also had a higher risk of 30-day CRNMB (2.0% vs. 1.1%; OR 1.74, 95%CI 1.57–1.93). Otherwise, elderly patients were less likely to experience 30-day recurrences (0.7% vs. 1.1%; OR 0.62, 95%CI 0.54–0.71) (Table 4).
Thirty- and ninety-day outcomes in the first 100,000 patients in the RIETE registry, according to age.
| N | ≥70 years | <70 years | Odds ratio (95%CI) | |
|---|---|---|---|---|
| PatientsN | 47,884 | 52,116 | ||
| 30-Day recurrent VTE | 907 | 329 (0.69%)‡ | 578 (1.11%) | 0.62 (0.54–0.71) |
| 30-Day major bleeding | 1278 | 836 (1.74%)‡ | 442 (0.85%) | 2.08 (1.85–2.33) |
| Sites of major bleeding | ||||
| Gastrointestinal | 410 | 276 (0.58%)‡ | 134 (0.26%) | 2.25 (1.83–2.77) |
| Hematoma | 361 | 276 (0.58%)‡ | 85 (0.16%) | 3.55 (2.78–4.53) |
| Intracranial | 142 | 86 (0.18%)† | 56 (0.11%) | 1.67 (1.19–2.34) |
| Retroperitoneal | 111 | 66 (0.14%)* | 45 (0.09%) | 1.60 (1.09–2.33) |
| Urinary | 102 | 71 (0.15%)‡ | 31 (0.06%) | 2.49 (1.64–3.81) |
| Vaginal | 34 | 9 (0.02%)* | 25 (0.05%) | 0.39 (0.18–0.84) |
| Hemoptysis | 24 | 12 (0.03%) | 12 (0.02%) | 1.09 (0.49–2.42) |
| Hemothorax | 25 | 9 (0.02%) | 16 (0.03%) | 0.61 (0.27–1.39) |
| Articular | 5 | 5 (0.01%)* | 0 | - |
| 30-Day non-major bleeding | 1527 | 936 (1.96%)‡ | 591 (1.13%) | 1.74 (1.57–1.93) |
| 30-Day all-cause death | 3703 | 2488 (5.19%)‡ | 1215 (2.33%) | 2.33 (2.14–2.46) |
| Fatal PE | 686 | 491 (1.02%)‡ | 195 (0.37%) | 2.76 (2.34–3.26) |
| Fatal bleeding | 245 | 179 (0.37%)‡ | 66 (0.13%) | 2.96 (2.23–3.93) |
| 90-Day recurrent VTE | 1554 | 582 (1.22%)‡ | 972 (1.87%) | 0.65 (0.58–0.72) |
| 90-Day major bleeding | 1789 | 1133 (2.37%)‡ | 656 (1.26%) | 1.90 (1.73–2.09) |
| Sites of major bleeding | ||||
| Gastrointestinal | 626 | 400 (0.84%)‡ | 226 (0.43%) | 1.93 (1.64–2.28) |
| Hematoma | 430 | 326 (0.68%)‡ | 104 (0.20%) | 3.43 (2.75–4.28) |
| Intracranial | 239 | 139 (0.29%)† | 100 (0.19%) | 1.51 (1.17–1.96) |
| Retroperitoneal | 136 | 81 (0.17%)† | 55 (0.11%) | 1.60 (1.14–2.26) |
| Urinary | 136 | 96 (0.20%)‡ | 40 (0.08%) | 2.62 (1.81–3.78) |
| Vaginal | 54 | 12 (0.03%)‡ | 42 (0.08%) | 0.31 (0.16–0.59) |
| Hemoptysis | 39 | 19 (0.04%) | 20 (0.04%) | 1.03 (0.55–1.94) |
| Hemothorax | 31 | 11 (0.02%) | 20 (0.04%) | 0.60 (0.29–1.25) |
| Articular | 7 | 5 (0.01%) | 2 (0.00%) | 2.72 (0.53–14.0) |
| 90-Day non-major bleeding | 2416 | 1446 (3.02%)‡ | 970 (1.86%) | 1.64 (1.51–1.78) |
| 90-Day all-cause death | 6486 | 4134 (8.63%)‡ | 2352 (4.51%) | 2.00 (1.90–2.11) |
| Fatal PE | 745 | 522 (1.09%)‡ | 223 (0.43%) | 2.56 (2.19–3.00) |
| Fatal bleeding | 343 | 235 (0.49%)‡ | 108 (0.21%) | 2.37 (1.89–2.98) |
| 90-Day arterial ischemic events | 195 | 118 (0.25%)‡ | 77 (0.15%) | 1.67 (1.25–2.23) |
Comparisons between patients<70 years and older than 70 years.
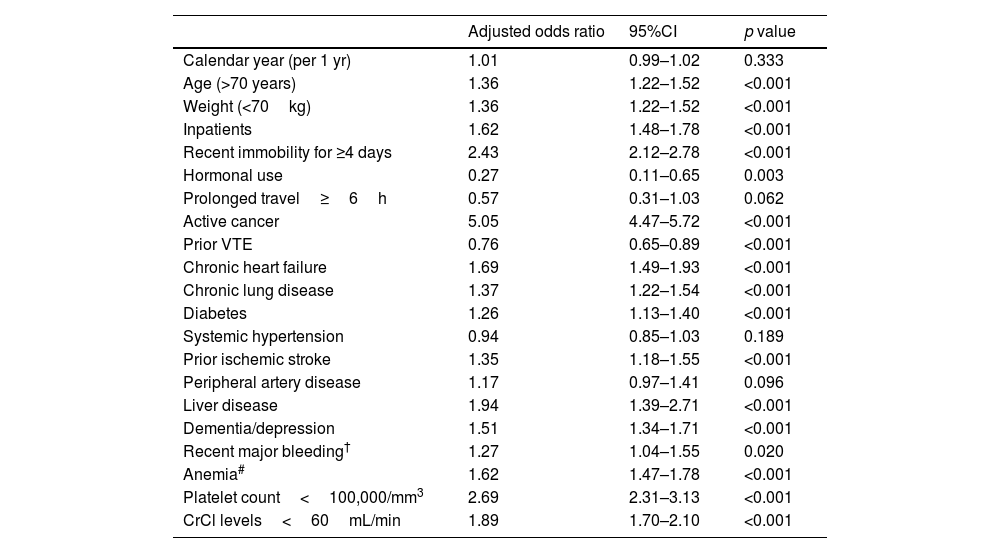
In the multivariable analysis, elderly patients had higher odds of 30-day all-cause mortality (adjusted OR 1.36, 95%CI: 1.22–1.52) (Table 5).
Multivariable model of predictors of 30-day all-cause mortality*.
| Adjusted odds ratio | 95%CI | p value | |
|---|---|---|---|
| Calendar year (per 1 yr) | 1.01 | 0.99–1.02 | 0.333 |
| Age (>70 years) | 1.36 | 1.22–1.52 | <0.001 |
| Weight (<70kg) | 1.36 | 1.22–1.52 | <0.001 |
| Inpatients | 1.62 | 1.48–1.78 | <0.001 |
| Recent immobility for ≥4 days | 2.43 | 2.12–2.78 | <0.001 |
| Hormonal use | 0.27 | 0.11–0.65 | 0.003 |
| Prolonged travel≥6h | 0.57 | 0.31–1.03 | 0.062 |
| Active cancer | 5.05 | 4.47–5.72 | <0.001 |
| Prior VTE | 0.76 | 0.65–0.89 | <0.001 |
| Chronic heart failure | 1.69 | 1.49–1.93 | <0.001 |
| Chronic lung disease | 1.37 | 1.22–1.54 | <0.001 |
| Diabetes | 1.26 | 1.13–1.40 | <0.001 |
| Systemic hypertension | 0.94 | 0.85–1.03 | 0.189 |
| Prior ischemic stroke | 1.35 | 1.18–1.55 | <0.001 |
| Peripheral artery disease | 1.17 | 0.97–1.41 | 0.096 |
| Liver disease | 1.94 | 1.39–2.71 | <0.001 |
| Dementia/depression | 1.51 | 1.34–1.71 | <0.001 |
| Recent major bleeding† | 1.27 | 1.04–1.55 | 0.020 |
| Anemia# | 1.62 | 1.47–1.78 | <0.001 |
| Platelet count<100,000/mm3 | 2.69 | 2.31–3.13 | <0.001 |
| CrCl levels<60mL/min | 1.89 | 1.70–2.10 | <0.001 |
Abbreviations: VTE, venous thromboembolism; CrCl, creatinine clearance.
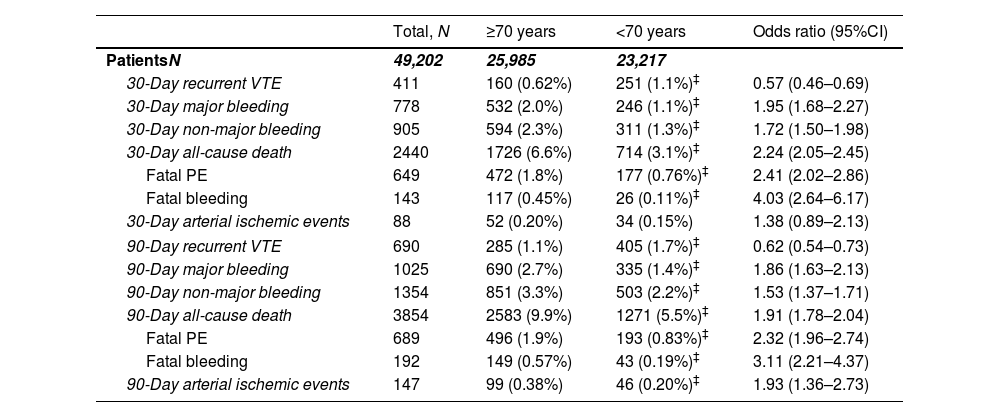
Among those patients with acute PE, we similarly observed an increased risk of both 30-day all-cause (6.6% vs. 3.1%; OR 2.24, 95%CI: 2.05–2.45) and PE-related mortality (1.8% vs. 0.8%; OR 2.41, 95%CI: 2.02–2.86) in older patients (Table 6).
Thirty- and ninety-day outcomes in patients with acute pulmonary embolism (N=49,202) in the RIETE registry, according to age.
| Total, N | ≥70 years | <70 years | Odds ratio (95%CI) | |
|---|---|---|---|---|
| PatientsN | 49,202 | 25,985 | 23,217 | |
| 30-Day recurrent VTE | 411 | 160 (0.62%) | 251 (1.1%)‡ | 0.57 (0.46–0.69) |
| 30-Day major bleeding | 778 | 532 (2.0%) | 246 (1.1%)‡ | 1.95 (1.68–2.27) |
| 30-Day non-major bleeding | 905 | 594 (2.3%) | 311 (1.3%)‡ | 1.72 (1.50–1.98) |
| 30-Day all-cause death | 2440 | 1726 (6.6%) | 714 (3.1%)‡ | 2.24 (2.05–2.45) |
| Fatal PE | 649 | 472 (1.8%) | 177 (0.76%)‡ | 2.41 (2.02–2.86) |
| Fatal bleeding | 143 | 117 (0.45%) | 26 (0.11%)‡ | 4.03 (2.64–6.17) |
| 30-Day arterial ischemic events | 88 | 52 (0.20%) | 34 (0.15%) | 1.38 (0.89–2.13) |
| 90-Day recurrent VTE | 690 | 285 (1.1%) | 405 (1.7%)‡ | 0.62 (0.54–0.73) |
| 90-Day major bleeding | 1025 | 690 (2.7%) | 335 (1.4%)‡ | 1.86 (1.63–2.13) |
| 90-Day non-major bleeding | 1354 | 851 (3.3%) | 503 (2.2%)‡ | 1.53 (1.37–1.71) |
| 90-Day all-cause death | 3854 | 2583 (9.9%) | 1271 (5.5%)‡ | 1.91 (1.78–2.04) |
| Fatal PE | 689 | 496 (1.9%) | 193 (0.83%)‡ | 2.32 (1.96–2.74) |
| Fatal bleeding | 192 | 149 (0.57%) | 43 (0.19%)‡ | 3.11 (2.21–4.37) |
| 90-Day arterial ischemic events | 147 | 99 (0.38%) | 46 (0.20%)‡ | 1.93 (1.36–2.73) |
Comparisons between patients<70 years and older than 70 years.
*p<0.05.
†p<0.01.
At 90 days after VTE diagnosis, 1789 (1.8%) patients had major bleeding (343 [19.2%] caused death) and 1554 (1.6%) patients had recurrent VTE (Table 4). Consistent findings were observed for age-related differences at 90 days in major bleedings (2.4% vs. 1.3%, OR 1.90, 95%CI 1.73–2.09), VTE recurrences (1.2% vs. 1.9%, OR 0.65, 95%CI 0.58–0.72) and all-cause mortality (8.6% vs. 4.5%, OR 2.00, 95%CI 1.90–2.11) (Table 4). In the subgroup of patients with PE, elderly patients had an increased risk of both all-cause mortality (9.9% vs. 5.5%, OR 1.91, 95%CI 1.78–2.04) and major bleeding (2.7% vs. 1.4%, OR 1.86, 95%CI 1.63–2.13) at 90 days, but a lower risk of 90-day recurrent VTE (1.1% vs. 1.7%, OR 0.62, 95%CI 0.54–0.73) (Table 6 and Fig. S2).
The incidence rate of other cardiovascular events at 90-days was very low (<1%) and mainly confined to elderly patients: overall, 195 patients developed arterial ischemic events (118 [60.5%] of these in those aged 70 years or older) (Table 4).
During the study period, there was a trend toward decreased mortality, VTE recurrences and major bleedings with similar tendencies for elderly and non-elderly adults in both the entire cohort and the PE patients (Fig. S3).
DiscussionIn our study of 100,000 patients with acute VTE, obtained from a large registry from over four hundred hospitals worldwide, we observed that elderly patients represented almost one in every two of. VTE patients. Elderly patients were predominantly female and more likely to have had an unprovoked event. They presented more frequently with signs of severity, including respiratory failure, myocardial injury and renal failure, but we did not observe differences in the rate of hemodynamical instability. While DOACs and advanced therapies (i.e., fibrinolytics, catheter-directed therapies, IVC filter, and ECMO) were uncommon, overall, in this cohort, they were used less frequently in elderly patients. Elderly patients showed an increased risk of 30- and 90-day all-cause mortality or major bleeding but a lower risk of VTE recurrences within the first 3 months. These findings were consistently observed in the subgroup of patients presenting with PE.
As expected, we found that elderly patients had different VTE risk factors and more comorbidities, including some degree of renal dysfunction in almost 60% of them. Elderly patients less likely presented with transient or reversible triggering risk factors; however, they had more concomitant disorders and persistent risk factor. These findings are consistent with prior data.4,12,29 The literature consistently indicates that elderly patients have more severe forms of clinical presentation of acute VTE.26 Barco et al. reported that age, and its interaction with other risk factors for VTE, appears to influence the presenting site of thrombosis, and this may explain differences in outcomes.11 Prior studies have reported that the risk of experiencing adverse outcomes in patients with VTE increases with age, and this circumstance can be partly explained by the comorbidity burden and lower cardiopulmonary reserve.4,6 In fact, the literature consistently confirms that age (mainly>70 years) is independently associated with increased morbidity and mortality.6,7,9,13,22,26,30
In our cohort, we observed that elderly patients initially showed more severe forms of VTE presentation (e.g., higher rate of acute PE and PE with respiratory failure or raised troponins). Furthermore, after adjustment for different prognostic conditions and other potential confounders, we found that age>70 years was independently associated with mortality. However, we failed to find age-related differences in either the proximal clot burden or the proportion of hemodynamical instability between elderly and non-elderly patients with acute PE. Nevertheless, both 30- and 90-day all-cause mortality in elderly patients were more than two times higher than in patients<70 years. Similarly, 30-day PE-related mortality was 2 to 3 times higher in older adults. These findings are consistent with previous studies showing that the risk of mortality is independently associated with older age and could be the consequence of a higher burden of co-morbidity, including a higher proportion of some prognostic preconditions in elderly adults.31 Surprisingly, we observed that the length of hospital stay was slightly lower in elderly patients, despite their comorbidities and poorer prognosis, with an average difference of almost 1 day. This finding could be explained by a higher short-term mortality rate among elderly patients.
We report that LMWH and oral anticoagulants were the most frequently used drugs for initial and long-term anticoagulation, respectively, with no clinically relevant differences between the age groups, consistent with prior prospective studies.32,33 Cefalo et al. noticed that treatment patterns did not differ between age groups in a cohort of 547 patients with acute PE.4 Comparable results were obtained in the Swiss registry (SWIVTER) of patients with acute VTE, where there was no difference in the use of reperfusion therapies between elderly and younger patients, despite a higher rate of massive PE in the elderly ones.26 In our study, we found that elderly patients were less likely to receive DOACs and underwent advances therapies, including IVC filter, reperfusion therapies and ECMO support. As renal function is a key consideration for DOACs administration, the lower use of DOACs in elderly patients could be explained by a significantly higher proportion of severe renal failure in older adults. It should be highlighted that a lower proportion of elderly patients received reperfusion therapies or ECMO, despite the lack of different rates of high-risk PE. These data may indicate that clinicians are less likely to offer these advanced therapies to elderly patients.
Moreover, we also observed that elderly patients had a twofold increase in the risk of major bleedings and CRNMB. We hypothesize that, as there were no differences in the mean doses of LMWH between elderly and younger patients, the higher rate of renal dysfunction in older patients (59% vs. 9%) played an important role in these results. A previous RIETE publication with an initial cohort of 13,011 patients with VTE, observed that elderly patients also presented an increased rate of bleeding complications.34
Lastly, we found that elderly patients were more likely to have unprovoked VTE. This is considered a risk factor for recurrent VTE,22,35,36 but our cohort presented a lower rate of VTE recurrences in elderly patients. One explanation might be that clinicians were more alert to maintaining anticoagulation for a longer time in elderly patients, according to the current treatment guidelines for patients with unprovoked VTE with no high risk of major bleeding.29,37–39 Furthermore, the majority of elderly patients in our cohort were women, while, conversely, the non-elderly patients were predominantly men; this circumstance may partly explain our finding, as previous studies have consistently found a lower risk of recurrence of VTE in women than in men.11,40
Our study has several limitations that should be addressed. First, our results should be treated with caution due to the limitations of observational studies. Second, we studied only the outcomes during a maximum of 90 days only, and no conclusions should be drawn beyond this period. Third, we included a wide range of locations (i.e., PE, proximal DVT, distal DVT, thrombosis on unusual sites) and forms of presentation (i.e., symptomatic, incidental, and asymptomatic) of VTE, and different natural evolutions are only to be expected. Lastly, there was no standardization of treatment, and so there were likely to be significant differences between countries and centers.
The main strength of our study is the multinational, multicentric prospective inclusion of unselected VTE patients in a real-world setting that provides a representative picture of the age distribution of VTE. Our data are obtained from RIETE, a solid platform with the largest multinational, observational cohort of patients with acute VTE available that allowed us to compare variables according to age groups with sufficient study power and describe their impact on clinical features, therapeutic strategies, and the short- and long-term outcomes of VTE. It is notable that all the identified clinically relevant age-related differences were highly statistically significant.
ConclusionIn summary, this large study reveals that age-related factors impact on clinical features, therapeutic choices and short- and long-term outcomes for acute VTE. Healthcare providers should take age-related considerations into account when determining the appropriate initial approach to managing VTE, because of their differences in short- and long-term prognosis.
All these findings provide real-world data on age-specific differences in clinical features and natural history of VTE and they may contribute to improving our comprehension of the age-related factors that influence the prognosis of patients with an acute VTE episode. Further research is needed, however, to elucidate the potential role of age in the effectiveness and safety of the various pharmacological and interventional therapies for VTE.
Coordinator of the RIETE RegistryManuel Monreal.
RIETE Steering Committee MembersPaolo Prandoni, Benjamin Brenner and Dominique Farge-Bancel.
RIETE National CoordinatorsRaquel Barba (Spain), Pierpaolo Di Micco (Italy), Laurent Bertoletti (France), Sebastian Schellong (Germany), Inna Tzoran (Israel), Abilio Reis (Portugal), Marijan Bosevski (R. Macedonia), Henri Bounameaux (Switzerland), Radovan Malý (Czech Republic), Peter Verhamme (Belgium), Joseph A. Caprini (USA), Hanh My Bui (Vietnam).
RIETE Registry Coordinating CenterS & H Medical Science Service.
FundingThis study received no funding.
Conflict of interestDr. Ido Weinberg declares:
Penumbra Inc. – Consultant
Magneto Thrombectomy Solutions – Consultant
Daiichi-Sankyo – Consultant
Arenal Medical – Consultant
We express our gratitude to SANOFI and ROVI for supporting this Registry with an unrestricted educational grant. We also thank the RIETE Registry Coordinating Center, S&H Medical Science Service, for their quality control data, logistic and administrative support and Prof. Salvador Ortiz, Universidad Autónoma Madrid, Statistical Advisor in S&H Medical Science Service for the statistical analysis of the data presented in this paper.
SPAIN: Adarraga MD, Alberich-Conesa A, Aibar J, Alda-Lozano A, Alfonso J, Amado C, Angelina-García M, Arcelus JI, Ballaz A, Barba R, Barbagelata C, Barrón M, Barrón-Andrés B, Bascuñana J, Beddar-Chaib F, Blanco-Molina A, Caballero JC, Castellanos G, Chasco L, Criado J, De Ancos C, Del Toro J, Demelo-Rodríguez P, De Juana-Izquierdo C, Díaz-Brasero AM, Díaz-Peromingo JA, Dubois-Silva A, Escribano JC, Falgá C, Farfán-Sedano AI, Fernández-Aracil C, Fernández-Capitán C, Fernández-Jiménez B, Fernández-Reyes JL, Fidalgo MA, Francisco I, Gabara C, Galeano-Valle F, García-Bragado F, García-Ortega A, Gavín-Sebastián O, Gil De Gómez MA, Gil-Díaz A, Gómez-Cuervo C, González-Munera A, Grau E, Guirado L, Gutiérrez J, Hernández-Blasco L, Jara-Palomares L, Jaras MJ, Jiménez D, Jiménez R, Jou I, Joya MD, Lainez-Justo S, Lecumberri R, León-Ramírez JM, Llamas P, Lobo JL, López-Jiménez L, López-Miguel P, López-Núñez JJ, López-Ruiz A, López-Sáez JB, Lorenzo A, Lumbierres M, Madridano O, Maestre A, Marchena PJ, Marcos M, Martín del Pozo M, Martín-Martos F, Maza JM, Mena E, Mercado MI, Moisés J, Monreal M, Morales MV, Navas MS, Nieto JA, Núñez-Fernández MJ, Olid M, Ordieres-Ortega L, Ortiz M, Osorio J, Otálora S, Otero R, Pacheco-Gómez N, Pagán J, Palomeque AC, Paredes E, Parra-Caballero P, Parra-Rosado P, Pedrajas JM, Pérez-Ductor C, Pérez-Pinar M, Peris ML, Pesce ML, Porras JA, Puchades R, Rivera-Cívico F, Rodríguez-Cobo A, Rosa V, Romero-Brugera M, Ruiz-Artacho P, Ruiz-Giménez N, Ruiz-Ruiz J, Salgueiro G, Sancho T, Sendín V, Sigüenza P, Soler S, Suárez-Fernández S, Tirado R, Torrents-Vilar A, Torres MI, Trujillo-Santos J, Uresandi F, Valle R, Varona JF, Villalobos A, Villares P, AUSTRIA: Ay C, Nopp S, Pabinger I, BELGIUM: Vanassche T, Verhamme P, Verstraete A, BRAZIL: Yoo HHB, COLOMBIA: Montenegro AC, Morales SN, Roa J, CZECH REPUBLIC: Hirmerova J, Malý R, FRANCE: Bertoletti L, Bura-Riviere A, Catella J, Chopard R, Couturaud F, Espitia O, Grange C, Leclercq B, Le Mao R, Mahé I, Moustafa F, Plaisance L, Sarlon-Bartoli G, Suchon P, Versini E, GERMANY: Schellong S, ISRAEL: Brenner B, Dally N, Tzoran I, IRAN: Sadeghipour P, Rashidi F, ITALY: Abenante A, Barillari G, Basaglia M, Bilora F, Bissacco D, Bortoluzzi C, Brandolin B, Casana R, Ciammaichella M, Colaizzo D, Dentali F, Di Micco P, Grandone E, Imbalzano E, Lambertenghi-Deliliers D, Negro F, Pesavento R, Poz A, Prandoni P, Scarinzi P, Siniscalchi C, Taflaj B, Tufano A, Visonà A, Vo Hong N, Zalunardo B, LATVIA: Paluga R, Skride A, Kigitovica D, PORTUGAL: Fonseca S, Marques R, Meireles J, Pinto S, REPUBLIC OF MACEDONIA: Bosevski M, Trajkova M, Zdraveska M, SWITZERLAND: Bounameaux H, Mazzolai L, UNITED KINGDOM: Aujayeb A, USA: Caprini JA, Weinberg I, VIETNAM: Bui HM.