Resectable non-small cell lung cancer (NSCLC) patients at diagnosis represents around 25–30%, and a complete surgical resection must be considered as a primary treatment. Adjuvant cisplatin-based chemotherapy (CT) should be recommended in patients involving lymph nodes (hilar and/or mediastinal), and can be indicated in tumors ≥4cm.1,2 In spite of that, the prognosis results poor, and overall survival at 5-year ranges from 80% (stage IA) to 40% (stage IIIA)(TNM 8th edition).3 Neoadjuvant CT achieves nearly identical finding of 5% improvement in 5-year overall survival versus adjuvant,4 although its use remains quite scarce in clinical practice worldwide.
Several major advances have recently been achieved in the field of early-stage resectable NSCLC, changing the standard of care of its management: adjuvant osimertinib,5 adjuvant immunotherapy,6,7 and preoperative chemoimmunotherapy.8
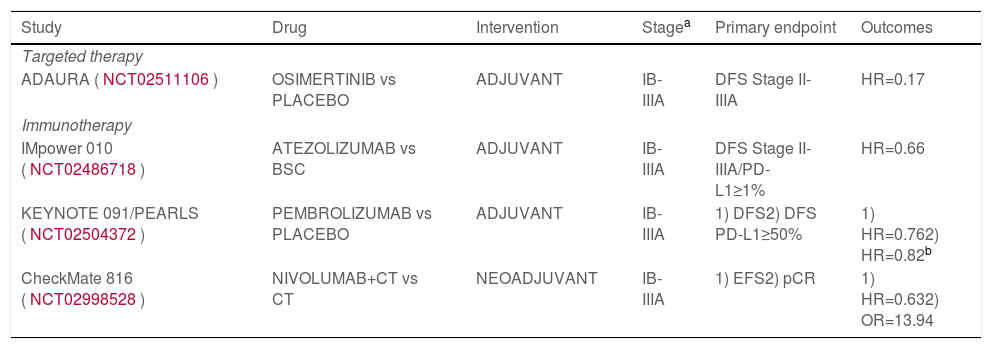
Over the past decade, biomarker-matched targeted therapies have been approved for clinical practice in the treatment of advanced NSCLC with significant improvement in survival.9 In the context of early-stage NSCLC, the most highlighted advance observed comes from the efficacy of adjuvant osimertinib for 3 years, a 3rd-generation EGFR-tyrosine kinase inhibitor (TKI), administered in a randomized, doble-blind, placebo-controlled phase III trial (ADAURA, NCT02511106), in patients harboring EGFR exon 19 deletions or exon 21 L858 mutation-positive stage IB-IIIA (TNM 7th edition) NSCLC patients, completely resected, with or without adjuvant CT. Primary endpoint of disease-free survival (DFS) in patients with stage II-IIIA was reached (hazard ratio (HR)=0.17, 99.1% confidence interval (CI) 0.11–0.26, P<0.0001), and also a significant DFS favoring osimertinib in the overall study population; central nervous system recurrence was also significantly lower. Its impact on overall survival is pending to be confirmed with longer follow-up1,5 (Table 1). In light of those results, molecular testing for EGFR in early-stage NSCLC must take part of our clinical practice. Osimertinib is approved by both the US Food Drug Administration (FDA) and the European Medicines Agency (EMA) as adjuvant treatment in completely resected NSCLC patients with stage IB-IIIA harbouring common EGFR-mutations irrespective of the use of adjuvant CT.1 In order to demonstrate the efficacy of osimertinib in earlier stages (IA2-IA3, TNM 8th edition), ADAURA2 trial (NCT05120349) is ongoing. Other targeted therapies are being assessed in different genotype directed clinical trials in adjuvant setting, such as LIBRETTO-432 phase III trial (NCT04819100) with adjuvant RET-TKI selpercatinib vs placebo in RET fusion-positive NSCLC patients, or ALINA trial (NCT03456076) with ALK-TKI alectinib in ALK fusion-positive NSCLC patients. In addition, several studies are ongoing in the neoadjuvant setting, like NeoADAURA phase III trial (NCT04351555) with osimertinib in combination or not with CT versus CT; and umbrella designed trials including a predictive biomarker panel and corresponding matched therapies: LCMC4 (NCT04712877), or NAUTIKA1 (NCT04302025).
Positive phase III studies of adjuvant or neoadjuvant targeted therapies or immunotherapies in resectable non-small-cell lung cancer.
| Study | Drug | Intervention | Stagea | Primary endpoint | Outcomes |
|---|---|---|---|---|---|
| Targeted therapy | |||||
| ADAURA (NCT02511106) | OSIMERTINIB vs PLACEBO | ADJUVANT | IB-IIIA | DFS Stage II-IIIA | HR=0.17 |
| Immunotherapy | |||||
| IMpower 010 (NCT02486718) | ATEZOLIZUMAB vs BSC | ADJUVANT | IB-IIIA | DFS Stage II-IIIA/PD-L1≥1% | HR=0.66 |
| KEYNOTE 091/PEARLS (NCT02504372) | PEMBROLIZUMAB vs PLACEBO | ADJUVANT | IB-IIIA | 1) DFS2) DFS PD-L1≥50% | 1) HR=0.762) HR=0.82b |
| CheckMate 816 (NCT02998528) | NIVOLUMAB+CT vs CT | NEOADJUVANT | IB-IIIA | 1) EFS2) pCR | 1) HR=0.632) OR=13.94 |
Abbreviations: BCS, best supportive care; CT, chemotherapy; cPR, pathological complete response; DFS, disease-free survival; EFS, event-free survival; HR, hazard ratio; OR, odds ratio; PD-L1, anti-programmed death-ligand 1.
Regarding other new approaches in development like immunotherapy, immune-checkpoint inhibitors (ICIs) promote host antitumor response, and outstanding advances have been achieved in metastatic and locally advanced NSCLC patients with this novel treatment strategy.1,9 Multiple adjuvant and neoadjuvant clinical trials are currently being carried out in early-stage NSCLC patients with encouraging and even satisfactory outcomes.
First, in the adjuvant setting, two randomized phase III studies have demonstrated significant efficacy. In IMpower010 study (NCT02486718), atezolizumab [anti-programmed death-ligand 1 (anti-PD-L1)] was administered for 1 year versus best supportive care in completely resected stage IB-IIIA (TNM 7th edition) NSCLC patients after cisplatin-based CT. Significant benefit in terms of DFS in PD-L1-expressing tumor cells (TC) ≥1% (SP263 assay) stage II-IIIA NSCLC was achieved with atezolizumab (HR=0.66, 95% CI 0.50–0.88, P=0.0039), although that benefit was observed specially in PD-L1 TC ≥50% (HR=0.43), and in all PD-L1 stage II-IIIA patients, but not in the intention-to-treat population.6 Positivity of circulating tumor (ct)DNA was a strongly prognostic factor for atezolizumab10 (Table 1). The FDA approved atezolizumab as the first ICI for adjuvant therapy. The second randomized positive phase III trial is KEYNOTE 091/PEARLS study (NCT02504372), administering pembrolizumab (anti-PD-1) vs placebo as adjuvant therapy in completely resected stage IB-IIIA (TNM 7th edition) NSCLC patients following adjuvant CT or not (investigator decision). The primary endpoint of DFS in the overall population was achieved (HR=0.76, 95% CI, 0.63–0.91, P=0.0014), but not the other dual primary endpoint that was DFS in PD-L1 ≥50% (22C3 assay) patients (HR=0.82, 95% CI, 0.57–1.18, P=0.14)7 (Table 1). Many other phase III clinical trials with other immunotherapeutic agents are ongoing: ANVIL (nivolumab, anti PD-1, NCT02595944), BR.31 (durvalumab, anti PD-L1, NCT02273375), MERMAID 1/2 (durvalumab, anti PD-L1, considering minimal residual disease (MRD), NCT04385368/NCT04642469), CANOPY-A (canakinumab, IL-1β inhibitor, NCT03447769); and their results will be available in the near future.11
On the other hand, the rationale for neoadjuvant immunotherapy may be stronger than adjuvant, enhancing T-cell priming and increasing expansion of antitumor T cells when bulk tumor and tumor antigens are still present during the therapy, offering an early opportunity to also treat micrometastasis. Several phase II clinical trials have been carried out with ICIs in monotherapy and in combination with CT in the neoadjuvant setting, but the first positive phase III trial is CheckMate 816 (NCT02998528), administering 3 cycles of neoadjuvant nivolumab in combination with CT versus CT before undergoing definitive surgery, in stage IB-IIIA (TNM 7th edition) NSCLC patients. Both primary objectives were achieved: significant differences in pathological complete response (Odds ratio=13.94, 99% CI, 3.49–55.75, P<0.001), and in event-free survival (HR=0.63, 97.38% CI, 0.43–0.91, P=0.005) favouring nivolumab with CT8 (Table 1). Recently, the US FDA has approved this indication and the EMA has validated its application, this is the first immunotherapy-based option authorized in the neoadjuvant setting for NSCLC patients. Several phase III studies with neoadjuvant ICI in combination with CT are ongoing, all of them including also the administration of adjuvant immunotherapy: CheckMate 77T (nivolumab, NCT04025879), KEYNOTE 671 (pembrolizumab, NCT03425643), IMpower 030 (atezolizumab, NCT03456063), or AEGEAN (durvalumab, NCT03800134). Additionally, different strategies are in development including new drugs and combinations.11,12
Lots of questions and novel needs arise in early-stage NSCLC patients: biomarkers testing (currently, at least EGFR-mutation and PD-L1 expression) looking for a better selection to improve outcomes; adjuvant or neoadjuvant treatment depending on some particular factors (no direct comparative studies yet); adequate duration of treatment and even for each patient; perioperative therapy in earlier stages; the role of new approaches in development; and more follow-up and overall survival data is still required in order to know the real impact of these strategies.
Finally, ctDNA and MRD by liquid biopsy may have a potential prognostic and/or predictive role in early-stage NSCLC patients that would help to determine therapeutic decision- making, and even duration of treatment. More prospective studies with larger patient populations and multiple early post-treatment timepoints are needed. Personalization according to ctDNA and MRD detection status may translate to improve patient outcomes.13,14
Important innovations recently introduced must be integrated into the multidisciplinary management of the early-stage NSCLC since they are already changing our clinical practice, and represent a clear paradigm shift in this disease. That is the right road to move forward in the cure of lung cancer, a challenging new era for our patients.