Obstructive sleep apnea (OSA), a common condition characterized by intrathoracic pressure reduction, sleep fragmentation and intermittent hypoxia due to repetitive upper airway obstructions during sleep, is a recognized cause of Hypertension (HTN).1 In clinical practice, however, it is difficult to prove causality, but the management of patients with co-morbid OSA and HTN may require an integrative approach. While the main treatments for OSA are well established regardless of presence of HTN (and may have a positive impact on blood pressure, BP, especially in those with resistant HTN),2 important questions related to the anti-hypertensive treatment deserve reflections: (1) Does OSA influence BP response to anti-hypertensive therapy?; (2) What is the best antihypertensive treatment in the OSA scenario? The first question was previously approached by our group in 2018.3 In an observational Cohort of 94 patients with HTN (55% of them with OSA) under a standard 30-day regimen of hydrochlorothiazide 25mg plus enalapril (20mg BID) or losartan (50mg BID), we evaluated the BP response up to 18 months of follow-up (no specific OSA treatment occurred during the investigation). Medical appointments were conducted regularly to perform medical adjustments in the antihypertensive medication regimens if necessary (in a blinded fashion). Compared with baseline, we did not observe significant differences between groups in 24-h BP, daytime systolic and diastolic BPs, or nighttime systolic BP at 6 and 18 months. The BP control rate at 24h (<130/80mmHg) was similar between the groups. Consistently, there were no differences in the number and class of antihypertensive medications prescribed during follow-up.3 Because patients in the aforementioned study used a combination of drugs, we speculated that multiple antihypertensive drugs acting in several pathways related to hypervolemia, the renin-angiotensin-aldosterone system, endothelial function, and sympathetic activation, among others, to mitigate the potential cardiovascular effects of OSA.3
The second question has a simple, but at the same time a challenge response: lessons learned from multiple studies revealed that continuous positive airway pressure (CPAP) per se is certainly not the best anti-hypertensive treatment. Overall, the modest BP lowering effects2 and the worst performance as compared to single drug therapy make this question apparently easy to address.4 However, in a recent retrospective analysis using insurance-based data, Revol and colleagues5 noted a significant reduction in the median cost of antihypertensive therapy and the use of two drug classes (calcium channels blockers, CCB and renin–angiotensin-system-acting agents) and a significant decrease in drug use for the same classes only in the OSA group. Despite the lack of data on OSA severity, BP values, and adherence to OSA therapy, these results suggest a specific effect related to OSA therapy, but the study design only generated hypothesis for future well-controlled prospective investigations.
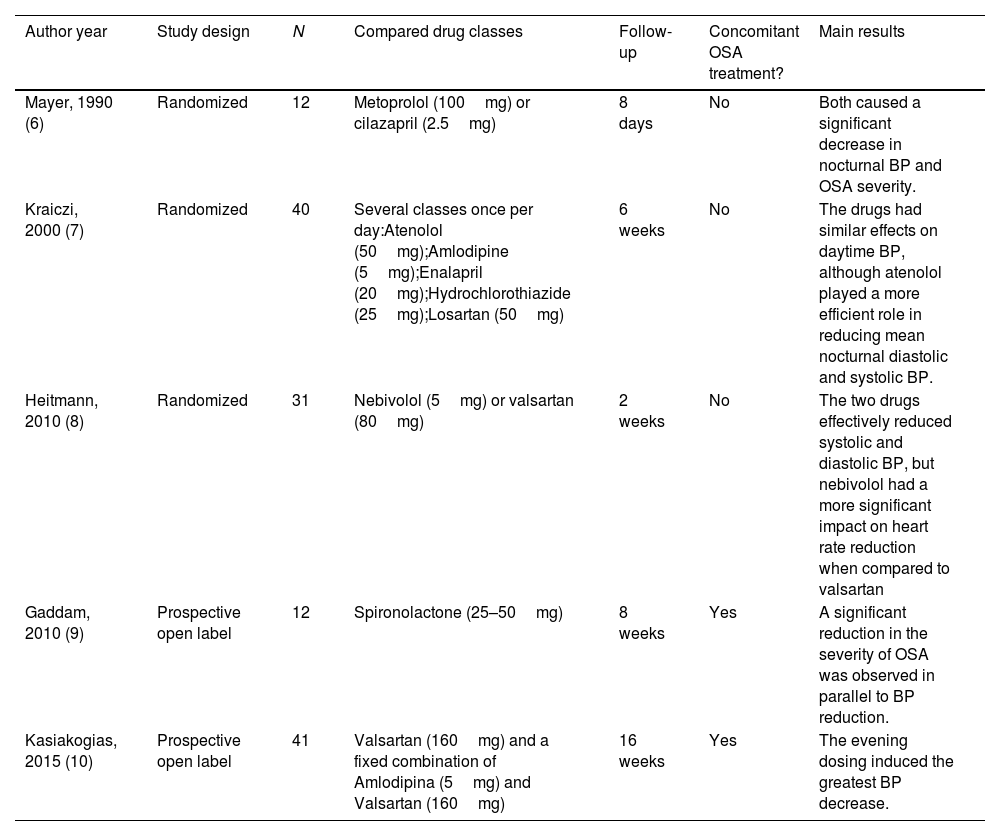
The complex history relies on the comparison of anti-hypertensive classes. Based on the aforementioned pathways, in theory some of them may have advantages over others. Previous studies have analyzed the effects of beta-blockers, angiotensin receptor blockers (ARBs), angiotensin-converting enzyme inhibitors (ACE), CCB and spironolactone are summarized on the following Table 1. Overall, the reported studies comprised small sample of patients followed by a short-term period.6–10 In addition, some of the studies did not have an active comparator or focused on the timing of anti-hypertensive intake. In two randomized studies,7,8 beta-blockers had some secondary advantages over the other classes but the aforementioned limitations preclude any definitive conclusions. Based on the available evidence, distinct Cardiology Societies did not determine any preference anti-hypertensive treatment with co-morbid OSA is present in patients with OSA.11,12
Summary on the studies that explored the effects of anti-hypertensive classes for patients with OSA.
| Author year | Study design | N | Compared drug classes | Follow-up | Concomitant OSA treatment? | Main results |
|---|---|---|---|---|---|---|
| Mayer, 1990 (6) | Randomized | 12 | Metoprolol (100mg) or cilazapril (2.5mg) | 8 days | No | Both caused a significant decrease in nocturnal BP and OSA severity. |
| Kraiczi, 2000 (7) | Randomized | 40 | Several classes once per day:Atenolol (50mg);Amlodipine (5mg);Enalapril (20mg);Hydrochlorothiazide (25mg);Losartan (50mg) | 6 weeks | No | The drugs had similar effects on daytime BP, although atenolol played a more efficient role in reducing mean nocturnal diastolic and systolic BP. |
| Heitmann, 2010 (8) | Randomized | 31 | Nebivolol (5mg) or valsartan (80mg) | 2 weeks | No | The two drugs effectively reduced systolic and diastolic BP, but nebivolol had a more significant impact on heart rate reduction when compared to valsartan |
| Gaddam, 2010 (9) | Prospective open label | 12 | Spironolactone (25–50mg) | 8 weeks | Yes | A significant reduction in the severity of OSA was observed in parallel to BP reduction. |
| Kasiakogias, 2015 (10) | Prospective open label | 41 | Valsartan (160mg) and a fixed combination of Amlodipina (5mg) and Valsartan (160mg) | 16 weeks | Yes | The evening dosing induced the greatest BP decrease. |
In conclusion, the best available evidence so far did not support any specific preference for the pharmacological anti-hypertensive treatment. Important to highlight that the evidence is limited, underscoring the need of well-designed, multicenter studies with large samples and long follow-up periods to expand our knowledge in the field. As suggested by a previous randomized study,4 combining treatment strategies seems to be the best option for improving sleep-related symptoms in parallel to decrease BP. Despite the significant controversies in the hypertension field related to the timing of anti-hypertensive intake,13,14 OSA patients might have more BP benefits using evening than morning dose, but definitive conclusions is lacking.
Conflict of interestsThe authors state that they have no conflict of interests.