Introduction
Video-assisted thoracoscopic surgery (VATS), dating from the early 1990s, has revolutionized the treatment of several thoracic diseases and conditions1. Pneumothorax is among those that have benefited most from the gradually increasing use of VATS, which provides a less invasive means to resolve a condition that is benign but of high prevalence among young patients2. Pneumothorax has become one of the most accepted indications for VATS3.
The standard initial treatment for spontaneous pneumothorax is thoracic drainage. However, if the problem is not resolved (persistent pneumothorax) or there is recurrence, surgical treatment should be considered4,5. The surgical techniques (standard thoracotomy, axillary thoracotomy and VATS) enable closure of the air leaks that cause the pneumothorax, wedge resection of the affected zone and/or pleurectomy6.
Spontaneous pneumothorax can be classified as primary (due to rupture of a subpleural bleb in a normal pulmonary parenchyma) or secondary (when the air leak occurs in a diseased lung)7. In both forms, an important causal role is played by blebs and bullae, which are present in a high percentage of patients and which can be detected during surgery8. VATS is therefore an ideal procedure for detecting such macroscopic lesions, and their resection by endoscopic linear cutter has become the treatment of choice9.
The present paper describes the indications for VATS in our hospital and the technique applied, as well as the complications and results observed over a three-year period.
Material and methods
Patients
A prospective study was carried out on 238 VATS interventions taking place over a period of 37 months. In 107 interventions (44%) the indication for surgery was primary or secondary pneumothorax.
Surgery was performed on 105 patients (78 men and 27 women) with a mean age of 28.78±13.96 years (range: 1578 years).
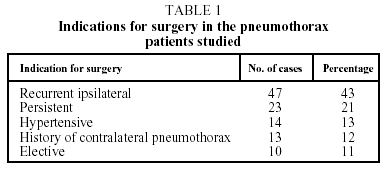
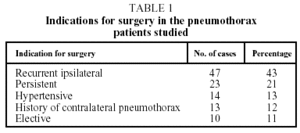
Indications for surgery were the following: recurrent ipsilateral pneumothorax (47 cases); persistent air leak for more than five days even after checking correct placement of and active drainage from the chest tube10 (23 cases); hypertensive pneumothorax (14 cases); a history of contralateral pneumothorax (13 cases); and elective surgery (10 cases). See Table 1.
Surgical technique
All procedures were performed with general anesthesia and selective intubation. The patient was in lateral decubitus position, resting on the side opposite the pneumothorax. After selective blocking of the affected lung, the surgeon made a first incision at the sixth or seventh intercostal space on the medial axillary line and a 10 mm trocar was inserted followed by a 0-degree optic. With video-assisted guidance two more trocars (diameters: 10 mm and 12 mm) were inserted into the chest cavity at the fourth intercostal space on the anterior axillary line and at the fifth intercostal space on the posterior axillary line, respectively. In no case was carbon dioxide used to aid in the formation of a thoracic space. After triangulation from the trocars, we explored the lung with an Endoscopic Blunt Dissector (BCD 10, Ethicon Endo-Surgery Inc., Cincinnati, Ohio, U.S.A.) to detect blebs or bullae causing air leaks. If adhesions were found, they were removed by electrocoagulation and scissors. An Endoscopic Articulating Linear Cutter (ETS - Flex 45, Ethicon Endo-Surgery Inc., Cincinnati, Ohio, U.S.A.) was used whenever lesions were found, but if none were visualized, a small portion of the pulmonary apex was biopsied for histopathologic study. The mean number of firing strokes was 2.7 per patient (range: 1-7).
The cavity was then washed with physiological saline solution to check for the continued presence of air leaks. Finally physical pleurodesis was performed by abrasion. Talc insufflation was never performed.
In all cases a chest tube (24 F) was inserted through the trocar situated on the medial axillary line and was connected to an aspirator of -20 cm H2O. In the cases of conversion to thoracotomy, two chest tubes were inserted.
The patients receiving VATS treatment were taken to the recovery room where they remained for a mean duration of 120 minutes (range: 60-180) before being taken to the ward. No patient required transfer to the postoperative intensive care unit.
Results
Of the 107 VATS interventions, 83 (77.6%) involved primary pneumothorax and 24 (22.4%) involved secondary pneumothorax.
There were no perioperative deaths. Conversion to posterolateral thoracotomy took place in three cases: one due to inadequate pulmonary blocking and two due to large pleuropulmonary adhesions detected on the mediastinal zone. These latter two cases were men with secondary pneumothoraces, in one of whom the pneumothorax was a recurrence.
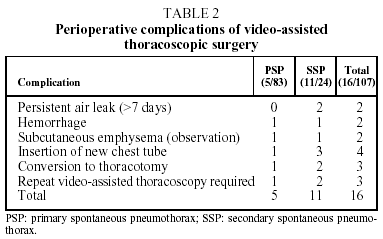
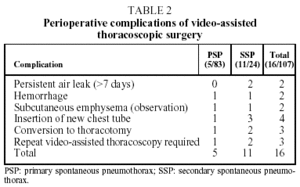
Perioperative complications developed in 16 cases. (See Table 2.) In 13 of these cases the complications were resolved through unassisted observation or by replacing the chest drainage tube. A second intervention was necessary in four cases (3.7%).
In two cases, an immediate postoperative intervention was performed due to hemorrhaging 6 and 18 hours, respectively, after the VATS. In one of these cases a transfusion of one unit of packed red blood cells was required. Both patients had undergone VATS for large mediastinal adhesions and in both cases the hemorrhage was located in an arteriole in the mediastinal region. The two other VATS-treated cases required re-intervention on the seventh and eighth day, respectively, to correct persistent air leak. In all four cases a posterolateral thoracotomy was performed.
During 93 interventions (86.91%) lung disease was identified as the cause or the presumed cause of the pneumothorax; thus resection was performed.
Chest tubes were removed a mean 1.96±1.01 days after surgery (range: 1-8 days). The mean postoperative hospital stay was 3.64 days (range: 3-12).
Two patients experienced a postoperative recurrence (ipsilateral pneumothorax <10%) following removal of the chest tube. They required no more than routine monitoring during out-patient appointments.
In the cases of two other patients, postoperative recurrences during the follow-up period were diagnosed when the patients came to our emergency room. These patients required more radical treatment. One woman with secondary pneumothorax suffered two episodes of partial basal ipsilateral pneumothorax eight and twelve months, respectively, after surgery. These two recurrences were successfully treated by chest drainage. The other patient, although young and presenting no risk factors, suffered a recurrence of 25% pneumothorax four months after surgery and required a thoracotomy.
Discussion
Spontaneous primary pneumothorax, traditionally treated by axillary thoracotomy, has become one of the most accepted indications for VATS2,9. In the case of secondary pneumothorax, VATS is gradually becoming a more accepted treatment11, although the presence of lung disease is a source of higher rate of complications12.
The cause of lesions in primary pneumothorax is not entirely understood. They may be the result of a break in the alveolar wall, causing air to leak into the pulmonary interstitial spaces and pleural viscera, forming small subpleural vesiculae13. In secondary pneumothorax, a demonstrated pulmonary disease would be the cause of the clinical picture. In the 24 cases of secondary pneumothorax in our study, the underlying pulmonary diseases were the following: chronic obstructive pulmonary disease/bullous emphysema (18 cases); infection in immunodepressed patients (4 cases); Langerhans cell histiocytosis (2 cases). There was a higher percentage of complications among secondary pneumothorax patients, as reflected in Table 2.
Spontaneous pneumothorax recurs after chest drainage treatment of a first episode in 20 to 25%14,15 of patients. In our study, the rate of recurrence after VATS treatment was 1.8%: two patients who suffered an ipsilateral pneumothorax. Only one of these patients received surgical treatment in a follow-up period limited to 14 months. In the series studied, the mean period of postoperative follow-up was 14.4 months (range: 2-36 months).
Some patients, for work-related or personal reasons, requested surgery at the first episode (elective surgery). This surgery was performed in 10 cases with no postoperative complications or recurrences. All 10 patients were operated on within a few days of the first episode and postoperative chest tubes were inserted.
The surgical technique used was insertion of three trocars (for proper triangulation) and resection by endoscopic stapler of the diseased zones (bullae or blebs), generally on the superior lobe. After checking for absence of air leaks, physical pleurodesis was performed. We performed neither talc insufflation (recurrence 1.79%) nor apical pleurectomy (recurrence 9.15%), although some authors report recurrence rates of postoperative pneumothorax above and below ours (7%). In some cases in our study, no chest tubes and/or thoracotomies were required. In our opinion talc, an adhesive agent of tested effectiveness, should be used only in patients with malignant pleural effusions. We did not use biological sealants, as we consider their high cost and debatable effectiveness for treating spontaneous pneumothorax16 does not make them a viable option.
In patients in whom no lung disease was evident (14 cases), a biopsy of the most apical part was performed by one or two firing strokes followed by physical pleurodesis. Sometimes subpleural blebs are difficult to see and a thorough inspection of the lung is needed. For such an exploration we use a blunt dissector which permits us to turn the surface of the parenchyma back, thus avoiding unnecessary traction which may lead to postoperative air leaks. This technique requires manual reexpansion of the lung by the anesthetist to provide a view of lesions which may be hidden by the atelectasis. One among those patients suffered a recurrence of pneumothorax (<10%) which only required out-patient management.
The mean duration of the postoperative hospital stay was 3.64 days. In the cases of pneumothorax treated by thoracotomy (axillary or posterolateral), the mean postoperative stay was 5.67 days, with 38% also requiring admission to a postoperative intensive care unit.
In conclusion, we consider that inserting a chest tube for drainage is the first measure to take on diagnosing spontaneous pneumothorax. In cases of contralateral or hypertensive pneumothorax, we opt for surgery, with VATS being our preferred technique. VATS is also the first technique we consider for treating persistent pneumothorax.
A thorough VATS exploration of the pulmonary parenchyma identifies lesions in a high percentage of patients because an endoscopic video camera offers a better view of the site than can be obtained by axillary thoracotomy.
The low rate of complications and the good long range results, in terms of short hospital stays and rapid functional recovery, make VATS our technique of choice in the treatment of both primary and secondary spontaneous pneumothoraces.
Correspondence: Dr. J.M. Galbis Caravajal.
Hospital General Universitario de Alicante.
Maestro Alonso, 109. 03010 Alicante. Spain.
E-mail: galbis_jos@gva.es
Mauscript received 6 October 2002.
Accepted for publication 12 October 2002.