Introduction
Noninvasive mechanical ventilation (NIV), defined as the use of a mechanical ventilation technique that does not require tracheal intubation, has been applied for several decades in patients with neurological diseases. In fact, most published reports of sustained clinical use of NIV involve such patients1-3, and NIV from negative pressure ventilators was widely used to treat respiratory insufficiency in patients with poliomyelitis between 1930 and 1960, leading to dramatic decreases in mortality. The publication of various noncontrolled trials describing cases successfully treated with NIV4-9 has meant that, for ethical reasons, hardly any controlled trials have been designed. Available reports indicate that NIV is able to reverse alveolar hypoventilation, improve quality of life and prolong survival in neurological patients, as well as provide other benefits. Nevertheless, it may be that such findings overestimate the benefits of NIV, given that negative results are seldom reported. The only controlled studies of NIV are those of Vianello et al10 and Shonhofer et al11. The former observed clinical and functional benefits in patients with Duchenne muscular dystrophy and the latter reported positive results in patients with chest restriction.
In this review, we discuss the underlying pathophysiology of NIV use in patients with neurological diseases, the general mechanisms of action of the technique and its indications and complications. We also analyze the mechanisms involved and results obtained with NIV in two neuromuscular diseases.
Pathophysiology of NIV in patients with neuromuscular diseases and in patients with chest restriction
Neuromuscular diseases
The alteration common to neuromuscular disease patients is respiratory muscle weakness, which varies highly according to the underlying disease. Differences arise because a) muscles are not affected to the same degree; b) respiratory muscle involvement may appear early or late; c) illness may be acute or may progress slowly; and d) muscle involvement may be reversible or recurrent. When respiratory muscle dysfunction is severe, respiratory insufficiency invariably develops and support ventilation becomes necessary.
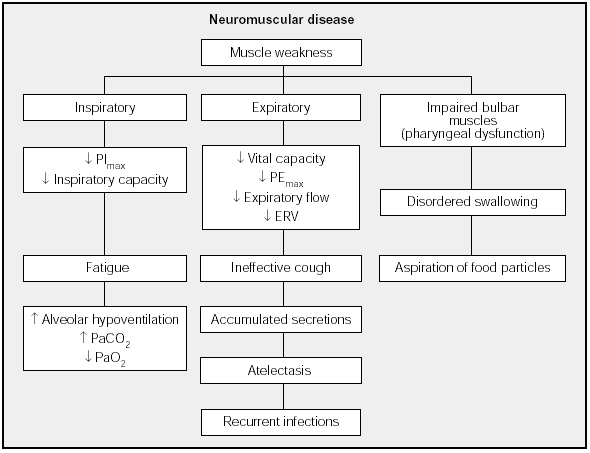
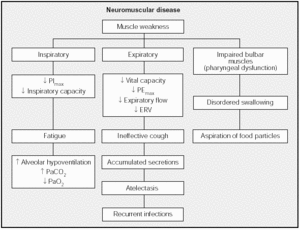
Weakness may affect three main muscle groups: inspiratory muscles (diaphragm, parasternal, scalene and accessory muscles); expiratory muscles (external intercostal and abdominal muscles); and muscles that innervate the upper airways (palatine, pharyngeal and genioglossal muscles)12 (Fig. 1).
Fig. 1. Physiological and clinical effects of weakness in respiratory muscles. PImax: maximal inspiratory pressure; PEmax: maximal expiratory pressure; ERV: expiratory reserve volume.
The end result of inspiratory muscle impairment is alveolar hypoventilation, with corresponding hypercapnia and hypoxemia. Expiratory muscle impairment, in turn, leads to an inefficient cough and retention of secretions. Finally, upper airway muscle impairment affects swallowing, leading to aspiration of saliva and food particles in the presence of an ineffective cough, a clinical picture that leads to recurrent respiratory infections. Such infections increase work of breathing, overloading inspiratory muscles that are already weakened by the underlying disease and triggering acute respiratory failure. However, the mechanisms that lead to chronic, progressive respiratory insufficiency in some neuromuscular diseases have not been clearly established. Two possible overlapping mechanisms have been suggested:
1. Inspiratory muscle fatigue, a result of increased work of breathing arising from diminished pulmonary distensibility due to microatelectasis and decreased distensibility of the thorax due to deformities secondary to muscle weakness. As a result, the load that must be borne by the muscles and their ability to bear it are imbalanced, such that the patient must develop greater pleural pressures (Ppl) to generate flow volume, given a diminished ability to generate maximal inspiratory pressure (PImax), with an increased Ppl/PImax ratio13-15. Decreased respiratory muscle strength in these patients has been clearly demonstrated by the reduction in both PImax and maximal expiratory pressure (PEmax). Less information is available on diaphragm function, however12,16.
2. Decreased respiratory muscle response. Neuromuscular disease patients experience frequent apneas and hypopneas during sleep due to the combination of respiratory muscle weakness and upper airway obstruction14. As a result, nocturnal hypoventilation leads to increased PaCO2 and CO2 retention, which in turn lead to decreased central respiratory drive. It has therefore been suggested that hypoventilation constitutes an adaptive mechanism aimed at decreasing effort of breathing, thereby preventing the development of inspiratory muscle fatigue17. The characteristic respiratory pattern of these patients is of rapid, superficial breathing.
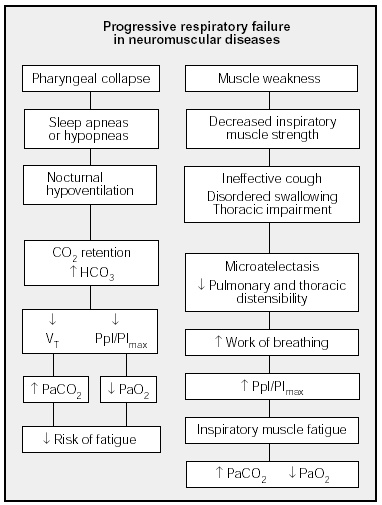
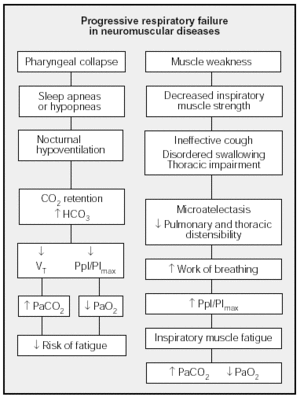
Figure 2 shows the mechanisms that have been said to be involved in hypercapnia in neuromuscular diseases.
Fig. 2. Mechanisms involved in respiratory failure in neuromuscular diseases. PImax: maximal inspiratory pressure; Ppl: pleural pressure; VT: tidal volume.
Chest restriction
Although neuromuscular diseases lead to structural changes in the thorax as a result of muscle weakness, primary deformities are also present in the chest, such as can be seen in idiopathic kyphoscoliosis or in the presence of thoracoplasty performed after lobectomy or pneumonectomy in tuberculosis. Such primary deformities may lead to respiratory failure if they are sufficiently pronounced.
In respiratory insufficiency due to severe kyphoscoliosis, a concomitant increase in thoracic impedance may contribute to greater respiratory loading and weakened respiratory muscles. Muscle weakness may be a sequela of poliomyelitis or a consequence of anomalous muscle insertion caused by thoracic deformity. Increased loading and decreased ability to generate force can lead to respiratory muscle fatigue. We observed severely reduced maximal transdiaphragmatic pressure (Pdimax) when we studied the relation between inspiratory muscle function and respiratory failure in a group of patients with severe kyphoscoliosis in comparison with an age-matched group of normal subjects13. In addition to lower PImax, we observed an increase in Ppl and Pdi deflections during relaxed breathing. Those increases led to higher Ppl/PImax and Pdi/Pdimax ratios, which approached figures that reflect respiratory fatigue. Pdimax correlated significantly with PaO2 (r=0.63) and PaCO2 (r=-0.76), and PImax was related to PaO2 (r=0.79) and PaC02 (r=-0.86). We also saw that Pimax rose in the six patients who underwent inspiratory muscle training for 12 months, with improvement coming after two months of training; moreover, hypercapnia was corrected in those who retained CO218. Such observations confirm the role of inspiratory muscle dysfunction in the development of respiratory insufficiency in these patients. Increased respiratory loading and decreased muscle strength may also be the mechanisms responsible for chronic respiratory insufficiency after thoracoplasty.
Apart from altered respiratory mechanics, another mechanism involved in respiratory insufficiency in kyphoscoliosis patients is hypoventilation and central or obstructive apneas during sleep14.
Mechanisms of action in NIV
The mechanisms by which NIV produces beneficial effects are not fully understood, although the following factors have been proposed to explain them15,17: a) respiratory muscle rest; b) improved central respiratory response to CO2; c) changes in pulmonary mechanics; and d) improved sleep architecture.
As previously mentioned, the first mechanism assumes that inspiratory muscles are in a state of "chronic" fatigue. Intermittent nocturnal ventilation allows muscles to rest and recover, with a consequent improvement in inspiratory muscle function, ventilation and arterial blood gases during the day. Although some authors19,20 have shown that muscles rest during NIV, others have nevertheless observed increased inspiratory muscle effort in patients whose alveolar hypoventilation has been corrected by NIV21,22.
The second mechanism of action postulates that nocturnal NIV prevents hypoventilation during sleep, resetting central response to CO2 and improving ventilation and gas exchange during the day22.
NIV has also been said to improve pulmonary function by recruiting atelectasic zones, increasing pulmonary distensibility and improving ventilation-perfusion ratios.
None of these mechanisms has been shown to be uniquely responsible for improving respiratory insufficiency during the day, and therefore part of the improvement has been attributed to the beneficial effects of NIV on sleep architecture and fragmentation. Disordered sleep associated with central and mechanical respiratory dysfunction aggravates changes in arterial blood gases, particularly during rapid eye movement (REM) sleep14. Moreover, the symptoms most commonly reported by these patients are daytime sleepiness, fatigue, morning headache, cognitive dysfunctions and dyspnea, mainly related to sleep fragmentation secondary to apneas. NIV largely corrects these problems, such that this mechanism of action would also be implicated in producing treatment benefits. Results from the few studies evaluating the effect of withdrawing NIV from patients in whom the treatment has corrected hypoventilation support this hypothesis. Thus, one week after withdrawal of NIV, nocturnal hypoventilation became aggravated and symptoms recurred, in the absence of lung function changes21. Similarly, Masa et al23 showed that suspending NIV for 15 days produced no changes in arterial blood gases, spirometry, lung volumes or maximal respiratory pressures measured during the day, even though there was severe deterioration in gas exchange during REM sleep a long with polysomnographic changes.
Clinical and functional effects of NIV
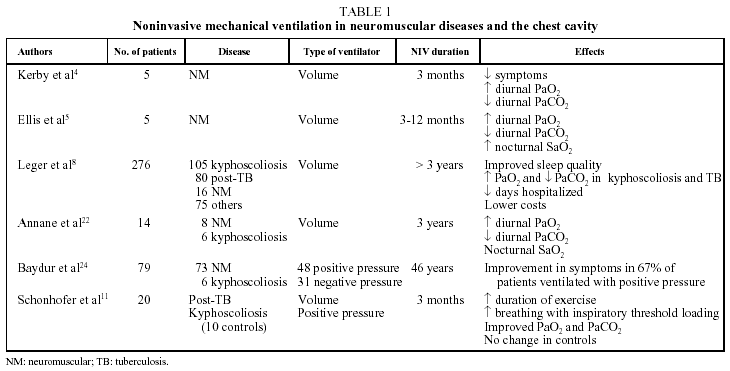
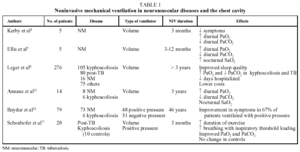
Table 1 summarizes the features of some of the most important studies published on the clinical and functional effects of NIV; among them, only that of Schonhofer et al11 included a control group. Many studies enrolled patients with both neuromuscular diseases and thoracic restriction, and most reported beneficial effects on both clinical and functional variables. Among the clinical gains were decreased daytime sleepiness, increased ability to perform activities of daily living, greater sense of well-being, a reduction in the number of hospitalizations and prolonged survival. The main functional benefits were improved or corrected diurnal hypoxemia and hypercapnia, improvement in nocturnal hypoventilation, and in come cases increases in maximal respiratory pressures.
Indications for NIV in neuromuscular diseases
NIV is prescribed mainly to treat increasing daytime hypercapnia in neuromuscular disease patients. Temporary application during respiratory infections or in patients who are frequently admitted for episodes of respiratory insufficiency have also been suggested. No benefit has been demonstrated for the prophylactic use of NIV, however, in patients without diurnal hypercapnia.
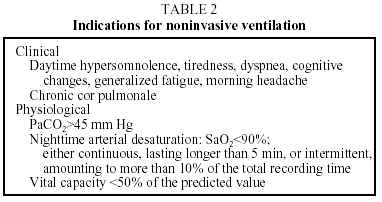
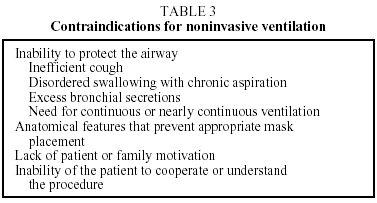
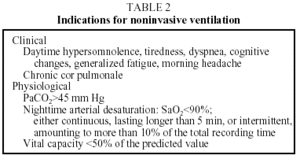
Indications for using NIV in patients with chronic respiratory insufficiency take into consideration the clinical and pathophysiologic characteristics1,3,17,25 shown in Table 2. Table 3 shows contraindications.
Complications of NIV
The complications of positive pressure NIV are usually mild, are generally related to the interface (air leaks, nasal pressure sores, nasal dryness and eye irritation) and can usually be overcome by changing the interface or undertaking fairly simple interventions. Less frequent complications are aspiration, pneumothorax and an inability to tolerate ventilation.
It is important to point out that one death due to respirator failure has been reported in a patient receiving NIV26, underlining the need to check respirators periodically.
Domiciliary NIV has been increasingly prescribed. A recent article on the current status of this procedure in Spain found that over 1,800 patients are ventilated at home27. We found no reports in the literature on the prevalence of domiciliary NIV in Latin America.
NIV in specific neuromuscular diseases
The two most prevalent nervous system diseases that give rise to respiratory insufficiency susceptible to treatment with NIV are amyotrophic lateral sclerosis (ALS), which affects motor neurons, and Duchenne muscular dystrophy, which affects the muscle.
Motor neuron diseases
Motor neuron loss in ALS leads to atrophy of the corresponding muscle, with consequent weakening of respiratory muscles. Early stages of disease are therefore generally asymptomatic and respiratory insufficiency usually manifests late, although it is the presenting symptom in approximately 5% of cases23,28,29. Muscle weakness becomes more marked as ALS progresses and degree of muscle involvement is the only parameter able to predict survival. Moreover, when muscle function becomes pronounced, the patient feels impending ventilatory failure. Although respiratory muscle deficits may manifest only during sleep initially, respiratory insufficiency invariably develops. At first, when symptoms are the result of changes in sleep architecture or nocturnal CO2 retention, the symptoms will be daytime somnolence, problems with concentration and morning headache in the absence of dyspnea. When respiratory insufficiency is present, however, dyspnea is usually among the symptoms and is due to marked diaphragm dysfunction30. If respiratory muscle impairment is the suspected cause of any of the aforementioned symptoms, that possibility should be carefully investigated. Although some authors have advised that assessment should include the measurement of respiratory muscle strength, particularly by means of tests that do not require volitional participation from the patient, such testing can be difficult to carry out and great variability has been observed in patients with established diaphragm muscle impairment31. Therefore, assessment will depend on the stage of disease. Early sleep disorders, once suspected, should be assessed by polysomnography to determine whether changes in gas exchange during sleep are due to obstructive apneas, which need only continuous positive airway pressure (CPAP) treatment, or to episodes of hypoventilation, which require NIV support. Polysomnography gives additional information on disease progression, as abnormalities only occur during REM sleep in early stages. When dyspnea is present, diurnal arterial blood gas measurement is imperative and either clinical or laboratory assessment of diaphragmatic function is advised30-32. The possibility that breathless patients should begin receiving ventilatory support without further examination has also been proposed17. Although NIV has been shown to prolong survival in ALS patients until advanced stages of the disease, when patients begin to feel extremely "locked in", contradictory reports of the effects of ventilatory support on quality of life have been made. While some authors have observed improved quality of life33-35, a study by Pinto et al36, comparing patients receiving NIV with a control group of patients who did not receive ventilation, found no evidence of quality of life gains, only longer survival in NIV patients who retained CO2. Those findings were confirmed by later studies37-39.
Unfortunately, adherence to NIV treatment is not good. Aboussouan et al39 observed that only 46% (18/39) of their patients with ALS tolerated treatment, with tolerance defined as the ability to use NIV at least 4 h without interruption during sleep. Bulbar symptoms, such as dysphagia, decreased the likelihood of tolerating NIV by half. The results of that study also demonstrated, moreover, that the risk of death was reduced 3-fold if NIV was tolerated.
It is important to point out that when respiratory failure presents in ALS, there is rapid overall deterioration, such that it is ethically necessary to discuss in a timely way the meaning of such deterioration and the therapeutic options available. A study of 38 patients with ALS found that most wanted as much information as possible about mechanical ventilation40 options. Nevertheless, the same study also demonstrated that opinions about resuscitation changed as the disease progressed, such that periodic review of the topic with patients is necessary. Moss et al41 studied 99 patients with ALS who had respiratory failure, finding that only 10% wanted invasive mechanical ventilation. Ninety percent of those who chose support ventilation, however, were satisfied with their decision.
Muscular dystrophies
Respiratory muscle impairment in Duchenne muscular dystrophy diminishes forced vital capacity. Alterations are evident in patients at an early age, generally at around age 12. Progression leads to diurnal CO2 retention between 18 and 20 years of age and the appearance of this sign is associated with approaching death from respiratory failure. NIV is recommended when patients report symptoms of respiratory insufficiency and daytime CO2 retention17, given that attempts to change the natural course of the disease by starting NIV when the patient has no respiratory symptoms have not produced benefits42,43. Measuring forced expiratory volume in one second has been suggested for monitoring muscular dystrophy patients but it seems more reasonable to use variables related to the restrictive nature of lung impairment and to decreased inspiratory muscle force, as has recently been demonstrated by Raggette et al44 in a study of 42 patients with primary myopathies. Those authors found that measuring inspiratory vital capacity (IVC) and PImax gives valuable insight into disease progression from the onset of sleep disorders until the appearance of diurnal respiratory insufficiency. According to their results, IVC less than 60% of the predicted value and a PImax less than 4.5 kPa predict the onset of sleep disorders, while an IVC less than 25% of the predicted value and a PImax less than 3.5 kPa predict the appearance of diurnal respiratory insufficiency44. Just as in ALS, early functional changes in breathing may manifest only during sleep, such that the patient should be asked about symptoms of nocturnal hypoventilation, morning headache, frequent awakening and daytime sleepiness. A base excess greater than 4 mm/l in arterial blood predicts significant nocturnal desaturation45. Some patients with Duchenne disease may present obstructive sleep apneas before developing hypoventilation due to respiratory muscle failure, and in them CPAP may be temporarily effective46. However, progression is inevitable and NIV will become necessary.
NIV will probably prolong survival in Duchenne disease patients who develop hypoventilation. Prospective controlled trials are lacking because of ethical constraints that prevent us from acquiring objective evidence. Nevertheless, the hypothesis of prolonged survival is supported by the findings of Vianello et al10; in their study 5 patients with Duchenne disease who accepted ventilation were alive after 2 years, whereas only one of 5 patients who refused ventilation was alive.
The efficacy of NIV varies in different studies. Leger et al8 described a series of 16 patients in whom one third continued to receive NIV while another third advanced to ventilation through a tracheostomy. A more recent study of patients receiving NIV, on the other hand, reported a one-year survival rate of 85% and a 5-year survival rate of 73%, suggesting that the progression to invasive ventilation may not be inevitable47. It may be that tracheostomy prolongs survival more than NIV once an ineffective cough develops and severe quadriplegia is present. Robert et al48 described a mean survival of 7 years in such circumstances. On the other hand, the number of hospitalizations due to pulmonary complications in Duchenne disease patients is lower in those receiving NIV than in those with tracheostomies49.
The quality of life of patients with Duchenne disease who receive NIV is not different from that of other age-matched controls who have other non-progressive diseases requiring NIV50, but the literature contains no studies comparing quality of life in these patients before and after initiating NIV. Most patients in these circumstances describe their quality of life as satisfactory50,51. Caregivers who work with such patients usually underestimate patient satisfaction with quality of life, and that is often given as the explanation for why some physicians do not propose NIV to Duchenne disease patients whose quality of life they assume will be unsatisfactory52.
In summary, even though most information on NIV in patients with neuromuscular or thoracic cavity diseases are based on uncontrolled studies, the many reports of beneficial effects and of improvement in the underlying pathophysiology support the use of NIV. Nevertheless, contraindications must be taken into account and invasive ventilation considered on an individual basis.
Correspondence: Dr. C. Lisboa.
Departamento de Enfermedades Respiratorias.
Pontificia Universidad Católica de Chile.
Marcoleta, 345, piso 4. Santiago. Chile.
E-mail: clisboa@med.puc.cl
Manuscript received 30 January 2003.
Accepted for publication 30 January 2003.