COPD is a highly prevalent but underdiagnosed disease, due to the limited availability of forced spirometry (FS) in primary care (PC). Microspirometers are inexpensive, easy-to-use devices that can measure FEV6 and FEV1/FEV6, and may help reduce underdiagnosis. The aim of this study was to validate the Piko-6 COPD screening device by demonstrating a good correlation with standard FS.
MethodsFS and Piko-6 determinations were made in 155 patients suspected of having COPD. The correlations, ROC curves, and Youden's index of both methods were compared, taking FS as the gold standard.
ResultsFEV1, FVC and FEV6 correlation coefficients and FEV1/FVC and FEV1/FEV6 ratios were 0.87 (CI 0.836–0.909), 0.729 (CI 064–0.795) and 0.947 (95% CI 0.928–0.961), respectively. The ROC curve for FEV1 determined by Piko-6 achieved an area under the curve of 0.86 (95% CI: 0.78–0.92). Youden's index with a cut-off point of 0.70 for FEV1/FEV6 was 0.97.
ConclusionsPiko-6 may be useful for COPD screening in PC. Measurements obtained with this device correlate well with those determined by FS, particularly the FEV1/FEV6 ratio. This, combined with its low cost and ease of use, may contribute to reducing COPD underdiagnosis, although its exact role in the diagnostic process remains to be determined.
La EPOC es una enfermedad de elevada prevalencia pero infradiagnosticada, debido a la escasa implantación de la espirometría forzada (EF) en atención primaria. Los microespirómetros, baratos y de manejo sencillo, que pueden medir FEV6 y FEV1/FEV6, podrían contribuir a reducir el infradiagnóstico. El objetivo del estudio ha sido validar el dispositivo Piko-6 para el cribado de la EPOC, demostrando una buena correlación con la EF convencional.
MétodosSe han realizado una EF y una determinación con Piko-6 a 155 pacientes susceptibles de padecer EPOC. Se han comparado las correlaciones, curvas ROC e índice de Youden con ambos métodos, considerando la EF como patrón de referencia.
ResultadosLos coeficientes de correlación de FEV1, FVC y FEV6 y los cocientes FEV1/FVC y FEV1/FEV6 fueron de 0,87 (IC 95%: 0,836-0,909), 0,729 (IC 95%: 064-0,795) y 0,947 (IC 95%: 0,928-0,961) respectivamente. La curva ROC para el FEV1 determinado por Piko-6 alcanzó un área bajo la curva de 0,86 (IC 95%: 0,78-0,92). El índice de Youden para el punto de corte de 0,70 del FEV1/FEV6 fue 0,97.
ConclusionesEl Piko-6 puede ser útil para el cribado de la EPOC en atención primaria. Sus determinaciones presentan buena correlación con las obtenidas mediante EF, especialmente el cociente FEV1/FEV6. Esto, junto a su bajo coste y facilidad de uso, puede contribuir a reducir el infradiagnóstico de la EPOC, aunque su rol exacto en el proceso diagnóstico está aún por determinar.
COPD is diagnosed in advanced stages, preventing the early implementation of an effective treatment that would minimize the mid- to long-term effects of the disease. The EPI-SCAN study1 estimated a 10% prevalence of COPD among subjects aged 40 to 80 years, and a rate of underdiagnosis of 73%.2 The COPD strategy of the National Health System proposes greater involvement of Primary Care (PC) and improved healthcare coordination.3
Forced spirometry (FS) with a bronchodilator test is required for diagnosis,2 and also predicts survival.4 Generalized use of FS in PC is a challenge for the National Health System, and the implementation of this procedure has come up against serious difficulties due to factors such as poor performance techniques and interpretation,5 thus contributing to underdiagnosis.6
The percentage of patients with suspected or previous diagnosis of COPD who have undergone FS in PC is low.7 In this setting, quality spirometries ought to be performed by trained, qualified personnel, but in reality, the situation is far from ideal8: FS is underused and the quality of the test is often unsatisfactory, particularly due to the difficulty in obtaining an accurate FVC.9
Various scientific societies have proposed recommendations, guidelines, and benchmarks for standardizing and improving the quality of FS.10–12 However, studies continue to show limited accessibility to the test and poor adherence to the proposed recommendations.13,14
COPD screening in smokers in PC is still not generalized.15,16 The most common recommendation is to perform FS in smokers aged 35–40 with a pack-year index (PYI) ≥10 who are symptomatic,17 but its usefulness in asymptomatic smokers or former smokers is more problematic. A study performed in Spain detected COPD in 20% of asymptomatic patients, but other authors have reported lower rates. Therefore, this technique should form part of the routine examination of subjects at risk.18
The variables required for the diagnosis of COPD are forced vital capacity (FVC), forced expiratory volume in 1 second (FEV1) and FEV1/FVC ratio. Given the difficulty of obtaining FVC, forced expiratory volume in 6s (FEV6) has been proposed as a valid alternative, so FEV6 and the FEV1/FEV6 ratio may replace FVC when portable devices are used.19
Microspirometers are simple devices that could facilitate the implementation of FS in PC, and constitute a valid alternative that, according to the ERS, should be investigated.20 At least 2 portable devices (COPD-6 and Piko-6) that can evaluate FEV1 and FEV6 are available. They are inexpensive, small, easy to use, and do not require calibration. These features could make them useful, at least in COPD screening in PC,21,22 although very few publications have evaluated their diagnostic validity compared to conventional spirometers in Spain.
The aim of this study was to determine the usefulness of the Piko-6 device for COPD screening, and to determine concordance between FEV1FEV6 and FEV1/FEV6 obtained with this device and FVC, FEV1, and FEV1/FVC obtained with FS. The study also aimed to identify the cut-off point of the FEV1/FEV6 ratio for the Piko-6 device that would rule out the presence of COPD in susceptible patients, and to assess whether FEV1 obtained by Piko-6 is useful for classifying patients according to the functional severity classification.
Materials and MethodsPatients with no previous diagnosis of COPD who met the criteria for COPD screening (age ≥40 years, PYI ≥10, and typical symptoms, such as cough, expectoration and dyspnea) were recruited at 2 PC centers. Patients who met the foregoing criteria were invited to participate in the study and, if they agreed, they were referred to the respiratory department for FS and microspirometry using the Piko-6 device (always in the same order). All patients with the foregoing criteria for COPD screening who agreed to participate in the study were included consecutively during the recruitment period until the desired number of patients was achieved.
Exclusion criteria were contraindication for performing spirometry and inability to correctly perform the Piko-6 and/or the spirometry maneuvers. Acceptability and repeatability criteria according to SEPAR Spirometry Guidelines 2013 were used, and patients with good and acceptable quality spirometries (A, B, C) according the spirometry quality criteria of these guidelines were included in the study.
Variables collected were: demographic data, symptoms, history of exacerbations, determinations of FEV1, FVC and FEV1/FVC with FS, and FEV1, FEV6 and FEV1/FEV6 with Piko-6; the subjective difficulty perceived by patients for performing each of the tests was also evaluated using a visual color scale graded from 0 to 10.
Statistical StudyThe sample size was calculated on the basis of the first 40 consecutive patients enrolled, estimating that the Piko-6 could have a sensitivity of 90% and specificity of 80% for the detection of obstruction, with a prevalence of obstruction of 40% and an alpha error of 5%. A sample size of 154 patients was required to obtain an accuracy of 90%.
Data were coded in an Excel spreadsheet and exported to SPSS 18.0, applying the tests appropriate to the type of variable studied. Differences were expressed as mean plus 95% confidence interval (95% CI). Quantitative variables were compared with the Student's t-test for paired samples. In the bivariate analysis with qualitative variables, the Chi-squared and Fisher's exact test were applied. Significance for all comparisons was set at P≤.05.
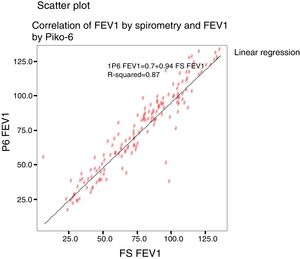
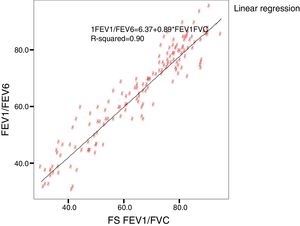
The Kappa index was used to evaluate the concordance between both devices in the detection of obstruction. The concordance and the relationship between the values was calculated with the intraclass correlation coefficient (ICC) and the Pearson (r) respectively, and are shown on scatter plots.
The validity and reliability of Piko-6 for detecting obstruction were determined using sensitivity and specificity values, positive predictive value, negative predictive value, and positive and negative odds ratios. We also calculated the area under the receiver-operating characteristic (ROC) curve of the FEV1/FEV6 ratio obtained with Piko-6 for discriminating obstruction, using as gold standard the FEV1/FVC ratio obtained by FS. The best cut-off point of the FEV1/FEV6 was determined using Youden's index, a statistic which is used to assess the best compromise between the sensitivity and specificity of a test.
ResultsWe included 155 patients, 111 men and 44 women, with an average age of 63±14 years (range 25–89). The percentage values of the parameters measured with FS and Piko-6 (mean and standard deviation) are shown in Table 1. FEV1 and FVC determined by FS were higher than FEV1 and FEV6 measured by Piko-6, but the differences between FEV1/FVC and FEV1/FEV6 determined by both devices were not significant. A contingency table was drawn up of patients diagnosed with obstruction by FS and Piko-6, using a<0.7 ratio in both cases. Table 2 shows the values derived from this test.
Mean Values and Differences of Parameters Determined by Spirometry and Piko-6.
| Spirometer (Mean; SD) | Piko-6 (Mean; SD) | P-value | Differences Spirometer-Piko-6 | |
|---|---|---|---|---|
| FEV1 | 77.5 (28.8) | 73.8 (29.1) | .000 | −3.7 (−5.4; −2) |
| FVC | 93.1 (21.3) | 87.7 (23.7) | .000 | −5.3 (−7.8; −2.8) |
| FEV1/FVC | 65.7 (17.2) | 64.9 (16.1) | .092 | −0.7 (−1.6; 0.1) |
Contingency Table of Number of Subjects Diagnosed With Obstruction by Spirometry and Piko-6.
| Forced Spirometry Reference Test | |||
|---|---|---|---|
| Piko-6 Diagnostic Test | Patients | Healthy | Total |
| Positive | 82 | 2 | 84 |
| Negative | 0 | 71 | 71 |
| Total | 82 | 73 | 155 |
| Value | CI (95%) | ||
|---|---|---|---|
| Sensitivity (%) | 100 | 99.4 | 100 |
| Specificity (%) | 97.2 | 92.8 | 100 |
| Validity index (%) | 98.7 | 96.6 | 100 |
| Predictive value+ (%) | 97.62 | 93.7 | 100 |
| Predictive value+ (%) | 100 | 99.3 | 100 |
| Prevalence (%) | 52.9 | 44.7 | 61.1 |
| Youden's Index | 0.9 | 0.9 | 1 |
|---|---|---|---|
| Likelihood ratio+ | 36.5 | 9.3 | 143.1 |
| Likelihood ratio− | – | – | – |
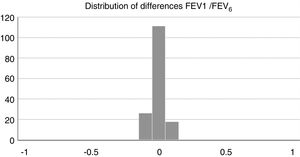
Concordance and correlation coefficients between the parameters were: FEV1 (FS) vs FEV1 (Piko-6): ICC 0.87 (95% CI; 0.83–0.90); FVC vs FEV6: ICC 0.72 (95% CI: 0.74–0.79); FEV1/FVC vs FEV1/FEV6: 0.94 (95% CI: 0.92–0.96). Scatter plots are shown in Figs. 1 and 2. The degree of correlation between Piko-6 and FS is excellent for all parameters studied, and always showed statistical significance (P<.0001). A skewness/kurtosis test was performed to represent the dispersion of Piko-6 values compared to FS. FEV1 showed kurtosis and skewness values of 6.19 and 2.65; FEV6, 5.65 and 2.52; and FEV1/FEV6, 17.5 and 4.09, respectively. Dispersion was small for all 3 parameters, and clearly lower for the variable FEV1/FEV6 (Fig. 3).
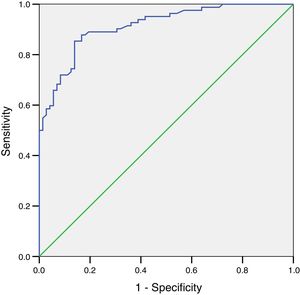
The ROC curve for FEV1 determined by Piko-6 showed an area under the curve of 0.86 (95% CI: 0.78–0.92) for detecting obstruction. Although this value is good, it did not reach the level of excellence achieved by the FEV1/FEV6 ratio (Fig. 4).
Table 3 shows sensitivity and specificity values, positive predictive value, negative predictive value, positive and negative odds ratios, and Youden's index for determining obstruction by Piko-6, using FEV1/FVC <0.7 obtained by FS as the gold standard. The best sensitivity/specificity compromise was obtained with the cut-off point of 0.70 (also used to rule out obstruction with FS). As shown in Table 3, the value of 0.70 provides the best indicators of test validity, between 96.6% and 100%. Concordance between both devices for determining obstruction using the Kappa index was 0.97 (standard error 0.018).
Sensitivity, Specificity, Predictive Values, Positive and Negative Odds Ratios for the Detection of Obstruction (FEV1/FVC <0.7 by Spirometry) for Different Cut-off Points for FEV1/FEV6 Measured by Piko-6 and Youden's Index.
| FEV1/FEV6 (Piko-6) | Sensitivity (%) | Specificity (%) | PPV+ (%) | NPV− (%) | OR+ | OR− | Youden's Index |
|---|---|---|---|---|---|---|---|
| <0.68 | 87.8 | 83.5 | 85.7 | 85.9 | 13.4 | 0.02 | 0.92 |
| <0.69 | 91.4 | 95.8 | 96.1 | 90.9 | 21.7 | 0.04 | 0.94 |
| <0.70 | 100 | 97.2 | 97.6 | 100 | 35.7 | 0.00 | 0.97 |
| <0.71 | 100 | 91.8 | 93.1 | 100 | 12.2 | 0.00 | 0.93 |
| <0.72 | 100 | 89.0 | 91.1 | 100 | 9.0 | 0.00 | 0.93 |
| <0.73 | 100 | 84.9 | 88.0 | 100 | 6.6 | 0.00 | 0.9 |
| <0.74 | 100 | 80.8 | 85.4 | 100 | 5.2 | 0.00 | 0.83 |
A secondary objective was to evaluate if FEV1 determined by Piko-6 could be used to classify severity in the case of obstruction. Table 4 shows the classification of patients according to FEV1 determined by Piko-6 and FS, and Table 5 shows the rates of concordance between both procedures with respect to COPD diagnosis and the severity of the obstruction. Concordance was found for diagnosis and severity in 87% of patients categorized according to the GOLD classification, and for diagnosis only, concordance was found in 12.3% (patients were classified correctly as having COPD [FEV1/FEV6 <0.7], but with a different level of severity according to FEV1 determined by FS), and in 1 case (0.6%), there was no concordance for either diagnosis or severity (the patient was classified as having COPD without having the disease, even when FS was used as the gold standard).
GOLD Classification of Patients, Depending on FEV1 and FVC Results Obtained Using FS and the Piko-6 Portable Device.
| GOLD by Spirometry | GOLD by Piko-6 | |||
|---|---|---|---|---|
| Frequency | % | Frequency | % | |
| Grade 0 | 73 | 47.1 | 73 | 47.1 |
| Grade I | 14 | 9 | 12 | 7.7 |
| Grade II | 37 | 23.9 | 34 | 21.9 |
| Grade III | 8 | 14.4 | 25 | 16.1 |
| Grade IV | 1 | 5.2 | 11 | 7.1 |
| Total | 155 | 100% | 155 | 100% |
Patients’ subjective difficulty of performing both tests was scored on a scale of 0–10. The mean difficulty for FS was 5.68 (SD 2.17), and 3.30 (SD 1.83; P<.0001) for Piko-6. In total, 73.9% of patients reported a difficulty of ≤5 with Piko-6, compared to 45.8% with FS.
DiscussionApproximately 1.5 million people in Spain have undiagnosed COPD.23 Moreover, many patients with a diagnosis of COPD in their medical history have never performed FS. Only 36% of PC physicians conduct spirometries in their asthma and COPD patients,24 leading to diagnostic errors, increased risk to patients, and rising costs of care.25
Underdiagnosis is a result of lack of awareness among the population26 and the scant use of spirometry in PC. Various scientific societies have published recommendations with minimum quality standards.27 The COPD consensus conference, sponsored by SEPAR, recommended performing FS in the PC setting in smokers over 40 years of age with respiratory symptoms. However, FS is still underutilized, and in many cases, poor quality results are obtained.28 Only 59.2% of health centers perform FS, 31% have not received any training, and 63.1% do not know how often spirometers should be calibrated,10 despite the consensus that PC is the most cost-effective setting for the early diagnosis and treatment of COPD, and the best for capturing the risk population.29
Microspirometers that can measure the FEV1/FEV6 ratio (assuming that FEV6 can often replace FVC) may be a good alternative, since it is easier to obtain FEV6 than FVC, thus reducing the variability of the technique: FVC is the main cause of FS variability, since the duration of the expiration differs among patients. This variability is reduced when FEV6 is used, as the duration of the expiration is always the same (6s).19,20
We included 155 patients in our study. The absolute and relative values of FEV1 and FVC determined by FS were significantly greater than FEV1 and FVC determined by Piko-6, but in contrast, no significant differences were observed between FEV1/FVC and FEV1/FEV6 ratios determined by both devices (P=.092). These are the parameters of interest, since they determine the existence of obstruction and are therefore useful in screening for COPD.
Very little literature is available on the clinical use of these devices. Rodríguez-Pascual et al.30 and Dal Negro et al.31 used the Piko-1, which cannot determine FEV6. Kaufmann et al.22 used the Piko-6, but their study design did not focus on the validation of the device to diagnose COPD. Frith et al.32 conducted a study designed to assess the usefulness of Piko-6 in the early diagnosis of COPD, but did not include a bronchodilator test. The study by Represas et al.,33 using the COPD-6, shows more similarities with our research, since the methodology and design are comparable. Other studies with contradictory results have been published: some found (despite limitations and certain premises) that microspirometers are useful for diagnosing COPD,34–36 whereas others conclude that results do not correlate well with FS determinations.37
Represas et al. found that absolute values of FEV1 and FVC determined by FS are significantly higher than those obtained by COPD-6.33 However, the FEV1/FVC ratio was significantly lower than that of FEV1/FEV6, although the predicted values used with each device were different (SEPAR and ECCS respectively),3 so no significant differences were observed in the analysis of the percentage differences from the expected value, with insignificant mean differences of even less than 1%.33
Obviously FEV6 is lower than FVC, since the latter accounts for the entire forced expiratory volume. This makes the mean FEV1/FEV6 ratio higher than FEV1/FVC, so the cut-off point used to determine obstruction in some studies cannot be 0.7, as pointed out by the manufacturer of the COPD-6. Thus, concordance using the same cut-off point for the 2 devices was only moderate. If the recommendations of the manufacturer are used, more than 40% of patients with obstruction according to FS would not have been detected with the COPD-6.33 These results contrast with the findings for Piko-6 in our study, which correctly diagnosed the majority of our 155 patients. In a study by Fritz32 comparing Piko-6 with FS, a cut-off point for the FEV1/FEV6 of <0.75 was used, yet despite this, sensitivity and specificity values of 81% (68–90) and 71% (63–79) were obtained. These figures are worse than those of our study, which are more comparable to those obtained by spirometry.
Similar findings have been reported in other studies designed to validate FEV6 as a replacement for FVC, to the extent that in several of them, the cut-off point for the FEV1/FEV6 ratio for the definition of obstruction was significantly higher than 0.7. If a cut-off of around 0.75–0.76 is used for the COPD-6, a better sensitivity/specificity ratio is obtained, making it useful for detecting obstruction. In contrast, in our study, Piko-6 offers high sensitivity and specificity with the cut-off point of 0.7, the same as that recommended for spirometry.
The contingency table of both techniques does not show significant differences: Piko-6 correctly diagnosed subjects who had FEV1/FVC <0.7 on spirometry. Sensitivity was 99% and specificity was >90%, so microspirometry with Piko-6 seems to be useful for the diagnosis of COPD. These values are higher than those reported by Represas33 with COPD-6, by Rodríguez-Pascual30 and Dal Negro31 with Piko-1, and by Kaufmann22 and Frith,32 who also used Piko-6.
The kurtosis test shows the scant difference in values compared to those obtained by FS, and the accentuated kurtosis in distributions, suggesting that the Piko-6 determinations correlate well with FS. Our results are also better than those of other authors using other microspirometers.
In our study, all correlations between FEV1, FEV6, and FEV1/FEV6 measured by Piko-6 and FEV1, FVC, and FEV1/FVC measured by spirometry had good linear regression lines, but the best result was obtained when FEV1/FEV6 was compared with FEV1/FVC (r=0.947), in line with those previously mentioned, confirming the comparability of both devices.
Other authors have also reported good correlations between the variables measured by microspirometers and FS. Represas et al.33 found an excellent correlation between the determinations obtained by COPD-6 and spirometry for FEV1/FEV6 (r=0.94, ICC: 0.93; P<.001), just below the value obtained in our study. Dal Negro31 found an excellent correlation between FEV1 determined by Piko-1 and FS (r=0.98; CI=0.979–0.986).
Once we established that Piko-6 is useful for detecting obstruction, we investigated the cut-off point of the FEV1/FEV6 ratio that would provide the best sensitivity and specificity values for the diagnosis of obstruction (FEV1/FVC <0.7). The cut-off point with the best sensitivity/specificity compromise was 0.7, with a Youden's index of 0.97. This is relevant, since it is the same cut-off used for FS to establish a COPD diagnosis, and as such, does not require adaptations or correction factors.
Represas,33 like Fritz32 with the Piko-6, concluded that for the COPD-6 it was advisable to use a cut-off point of between 0.75 and 0.80 for a diagnosis of COPD. However, while a higher result would rule out obstruction with acceptable reliability, a lower result would necessitate a spirometry test, since specificity was low.20 In conclusion, our study is the only one to determine 0.7 as the best cut-off point for FEV1/FEV6 for the diagnosis of COPD with Piko-6, without requiring corrections or adjustments. This may be because in our study, all tests were conducted by trained personnel, and the Piko-6 determination was performed after the spirometry, that is to say, under the effects of the bronchodilator.
A ROC curve was plotted for the FEV1/FEV6 variable with Piko-6 using the 0.7 cut-off. The curve result of 0.99 indicates that the reproducibility of the determinations compared to spirometry for detecting obstruction is excellent. Moreover, the Kappa index for concordance between the determinations obtained by both procedures was 0.97 for the comparison of FEV1/FEV6 and FEV1/FVC, and the OR tends toward infinity, indicating the validity of the diagnostic test.
Having demonstrated the usefulness of the technique for diagnosing obstruction, we also assessed if it was useful for evaluating the severity of the obstruction and for classifying patients using FEV1 according to the GOLD classification of functional severity.38 Most patients (135; 87%) were correctly classified, 12.3% were incorrectly classified, and in 1 case, a patient was diagnosed with COPD despite absence of the disease. Patients with no functional changes were correctly classified, and for this reason, the device seems to be useful for ruling out COPD, but Piko-6 overestimates functional involvement, since FEV1 values with Piko-6 are lower than with FS. The greatest differences between Piko-6 and FS occur with lower FEV1 and FEV6 values, so it may be possible to identify a FEV1 cut-off above which an FS will not be needed in patients with few symptoms, i.e., the patient population that GesEPOC and other consensus documents recommend be diagnosed and monitored in PC.39 However, larger studies are needed to confirm this possibility. Our group has launched a multicenter study, funded with a SEPAR grant, for this purpose.
Finally, we assessed the subjective difficulty of patients in performing both maneuvers (microspirometry with Piko-6 and FS), and found that patients find it easier to perform microspirometry than FS, perhaps because fewer maneuvers are required, the nozzle diameter is smaller, and expirations are shorter. The greater ease reported by patients is very important, as it may lead to a wider implementation and use of Piko-6 in PC than so far seen with spirometry.
A possible limitation of this study is the fact that both the FS and the determinations with the Piko-6 were conducted in the respiratory medicine setting to avoid bias from the bronchodilator component (the determinations with Piko-6 were always conducted after the FS with bronchodilator). Our working group is currently finalizing a study comparing the quality of determinations obtained with microspirometers and spirometers in health care levels in PC and respiratory medicine departments, and the results will soon be published.
In conclusion, the Piko-6 device may be useful for COPD screening in PC, as it shows good correlation with the FS, especially for the FEV1/FEV6 ratio, a finding also reported by Jing et al. in their meta-analysis published in Chest.19 This, along with the low cost, ease of use, rapid performance, and good acceptance of these devices by patients, can help reduce the underdiagnosis of COPD and the number of referrals to second level centers for FS. Although the exact role of microspirometers in the diagnostic process of COPD is yet to be determined, these devices may be useful in patients with moderate/severe obstruction who are, as we have already pointed out, the patients that should be diagnosed in PC.40
AuthorshipHidalgo Sierra V: study design, recruitment and drafting of the manuscript.
Hernandez Mezquita MA: design, coordination, supervision of results and drafting of the manuscript.
Palomo Cobos L: methodological review and statistical study.
García Sánchez M: patient recruitment.
Diego Castellanos R: patient recruitment of patients and performance of spirometries.
Jodra Sánchez S: performance of spirometries and manuscript revision.
Cordovilla Pérez R: literature search and manuscript revision.
Barrueco Ferrero M: study design, team coordination, supervision of results and drafting of the manuscript.
Conflict of InterestsThe authors state that they have no conflict of interests.
Please cite this article as: Hidalgo Sierra V, Hernández Mezquita MÁ, Palomo Cobos L, García Sánchez M, Diego Castellanos R, Jodra Sánchez S, et al. Utilidad del dispositivo portátil Piko-6 para la detección precoz de la enfermedad pulmonar obstructiva crónica en atención primaria. Arch Bronconeumol. 2018;54:460–466.