Physical activity (PA) is a significant clinical dimension in COPD, but no useful tools are available to determine this variable in routine clinical practice.
ObjectiveTo create a simple, easy-to-use, specific questionnaire to detect PA deficits.
MethodA multidisciplinary panel of COPD experts was formed to review PA, its determinants, and measuring methods. The methodology for selecting specific dimensions and items was agreed in rounds, and the aspects to be included in the preliminary version were determined. The questionnaire structure was defined according to applicability of these aspects in clinical practice. Agreements were reached by consensus of the members.
ResultsA total of 148 items were reviewed, of which only 3 were directly selected. It was decided that the questionnaire should evaluate the intensity (low, moderate, or intense), amount, and frequency of PA, and inactivity or sedentary lifestyles. It also gathers information on the profile of inactive patients, and includes a measure of impact, defined as the patient's perception of their expectations regarding activity, their personal experience, characteristics of their environment, and their personality. The questionnaire is divided into 2blocks, one aimed at quantifying PA, and the other at collecting data for defining the profile and impact in patients with low PA only.
ConclusionThe SAQ-COPD is a simple, short, specific questionnaire, designed to evaluate PA in COPD patients in clinical practice.
Aunque la actividad física (AF) es una dimensión clínica relevante en la EPOC, no existen instrumentos útiles en la práctica clínica habitual.
ObjetivoCrear un nuevo cuestionario específico, sencillo y de fácil aplicación que detecte el déficit de AF.
MétodoSe creó un panel multidisciplinar de expertos en EPOC y se revisó el estado de la cuestión sobre AF, sus determinantes y métodos de medida. Se consensuó la metodología de selección de dimensiones e ítems específicos por rondas, definiendo las dimensiones e ítems sobre los que formar la versión preliminar. La estructura del cuestionario fue definida de acuerdo con su aplicabilidad en la práctica clínica. Los acuerdos se alcanzaron por consenso de los miembros.
ResultadosSe revisaron un total de 148 ítems, de los que solo fueron seleccionados directamente 3. Se definió que el cuestionario debía evaluar la intensidad (baja, moderada o intensa), cantidad y frecuencia de AF, así como la inactividad o sedentarismo. También ofrece información sobre el perfil del paciente con baja actividad e incluye una medida de impacto, definido como la percepción del paciente respecto a sus expectativas de actividad, lo que abarca su experiencia personal, características de su entorno y personalidad. El cuestionario queda dividido en 2bloques: una herramienta destinada a cuantificar la AF y una parte informativa, solo para los pacientes con baja AF, destinada a definir su perfil e impacto.
ConclusiónEl SAQ-COPD es un cuestionario específico, breve y sencillo, para evaluar la AF en pacientes con EPOC, que se ha definido para que sea aplicable en la práctica clínica.
Physical inactivity is the fourth most important risk factor for global mortality and is estimated to cause 5.5% of deaths worldwide.1 Given its importance, physical activity (PA) is actively encouraged by the World Health Organization,2 which considers it to be one of the most beneficial health measures.3 There is, then, a huge interest in the development of simple, reliable, and reproducible tools for the assessment, control and monitoring of the level of PA in clinical practice.
Chronic obstructive pulmonary disease (COPD) limits PA in patients,4,5 even in early stages of the disease.6,7 Physical inactivity is one of the most important predictors of mortality in COPD patients,8,9 and is associated with a high risk of hospitalization and readmission.10 Inactivity is not only a result of functional respiratory impairment, but depends also on other factors,11 including dyspnea, pulmonary hyperinflation, age, and peripheral muscle weakness.11 Factors which have received less attention include comorbidities, sociodemographic factors (race, socioeconomic status, education level, etc.) and lifestyle (smoking, use of alcohol, day of the week, etc.), and even the patient's own engagement and expectations regarding their current disease status can have an impact on PA.
PA can be measured using motion sensors or questionnaires. Motion sensors mainly include step counters (pedometers) or body acceleration monitors (accelerometers). These devices record the number of steps taken in a certain time period, distance covered, activity pattern, estimation of energy expenditure or intensity, and PA level. Motion sensors are more precise, but are less accessible for daily clinical practice.12,13 Compared to these devices, questionnaires are a simple, inexpensive way of evaluating PA, although they are subjective and vary widely with regard to data collected, survey period, communication of results, and other aspects.12–15 Many PA questionnaires are not specific for COPD patients, or have a limited discriminatory capacity to detect changes or early alterations. They do not distinguish between involuntary and voluntary or adaptive inactivity, nor are other areas of PA considered, such as social barriers and cultural or motivational factors, so they do not provide a detailed profile of the inactive patient that would help lay the basis for a therapeutic intervention. Moreover, most available questionnaires are too long and complex to be administered in a busy health center.
The aim of this study was to create a specific questionnaire for COPD patients that would be simple, easy and relatively quick to administer, and would detect physically inactive patients, thus providing the doctor with additional information for distinguishing inactivity as a personal choice from voluntary-adaptive or functional incapacity-related inactivity, while taking into account the different barriers. Below, we describe the working methodology and the first proposal for the SAQ-COPD (Spanish Activity Questionnaire in COPD) questionnaire.
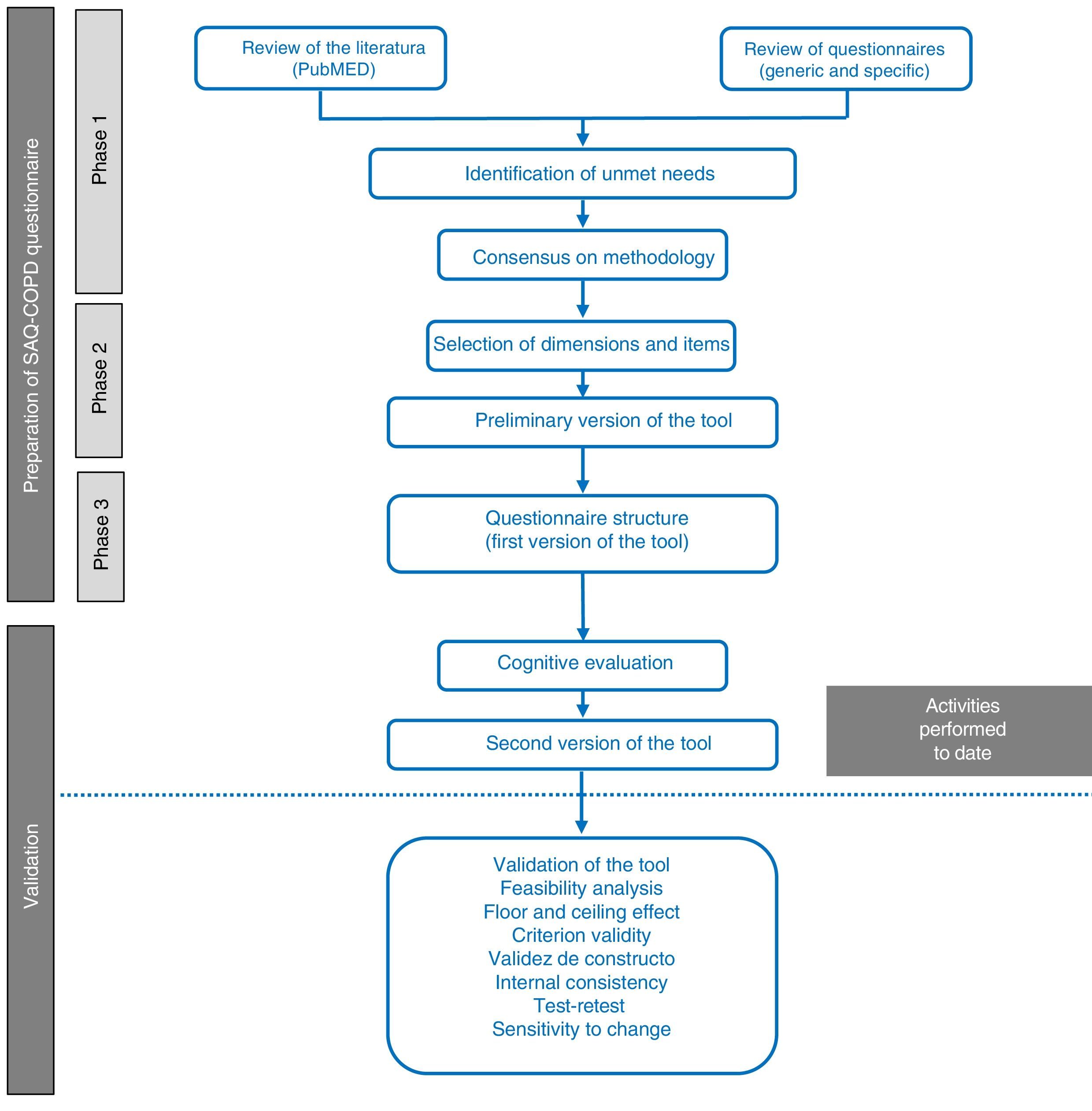
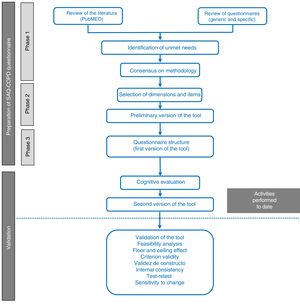
MethodologyTo create this new physical activity questionnaire, a working group was formed, consisting of a multidisciplinary panel of experts in the management and treatment of COPD that included 7 respiratory medicine specialists, a family medicine specialist, and a specialist in physical medicine and rehabilitation. The new questionnaire was developed in 3 phases (Fig. 1). The resulting questionnaire underwent cognitive validation.
Stages in the process of drafting and validating the SAQ-COPD questionnaire. The questionnaire was drafted in 3 phases: (1) review of the literature and identification of unmet needs; (2) selection of dimensions and items, and (3) structure of the preliminary version of the questionnaire. Cognitive validation is also included in this article.
This first phase had 2 objectives: (1) to review the current thinking about PA and its determinants in clinical practice, and to review the existing measurement methods and needs not met by previous questionnaires; and (2) to agree on the methodology for selecting dimensions and items for the first draft of the tool. A non-systematic literature search was performed in PubMed with the aim of identifying the determinants of PA and the most important questionnaires, in the opinion of the panel members, in order to select dimensions and items that could serve as the basis for the construction of a questionnaire. Questionnaires were considered if they were brief and validated for use in COPD, or had been used in international studies. Consensus was reached among all members of the panel using a methodology of selection by rounds (Delphi method) followed by discussion of the result.
Phase 2: Definition of Dimensions and Questionnaire ItemsThe aim of this phase was to define the dimensions and to select the variables that would shape the preliminary version of the questionnaire, and to precisely define which aspects should be measured (amount of PA, inactivity profile, and impact).
Using the selected questionnaires, a list of variables, classified as quantitative or qualitative and according to the dimensions analyzed (general activity, domestic activities, leisure, care of others, and self-care), was drawn up. The working group reviewed an initial selection of variables derived from these questionnaires. To evaluate the degree of agreement among the panel members, each item was rated on a 1–9 Likert scale, in which scores were grouped as 1–3 (“very strongly disagree”), 4–6 (“agree”), and 7–9 (“very much agree”). Items were accepted as candidates for inclusion in the new tool if at least 60% of panel members rated them as “very much agree”. Items rated “agree” or “very much agree” by 50%–60% of panel members were submitted for another round and subsequent discussion. All other items were discarded. Arbitrary thresholds for selecting items were previously agreed upon by the Scientific Committee. Panel members were also asked if the tool should be quantitative or qualitative, and if a technological measurement should also be included.
Phase 3: Structure of the SAQ-COPD QuestionnaireThe structure and presentation of the questionnaire in terms of its applicability in clinical practice were defined in the last phase, and the preliminary version of the tool was drafted.
Cognitive ValidationA linguistic comprehension analysis was performed to confirm that each item was comprehensible, or if rewording was necessary. The factors evaluated were: words that might be unfamiliar or ambiguous, the clarity of the item, the time needed to complete the item, and the time needed to complete the dimension. It was determined that items that 30% or more of patients found hard to understand would require modification and re-assessment. Data were collected on age, sex, educational level, employment status, and severity of the disease for the characterization of the sample.
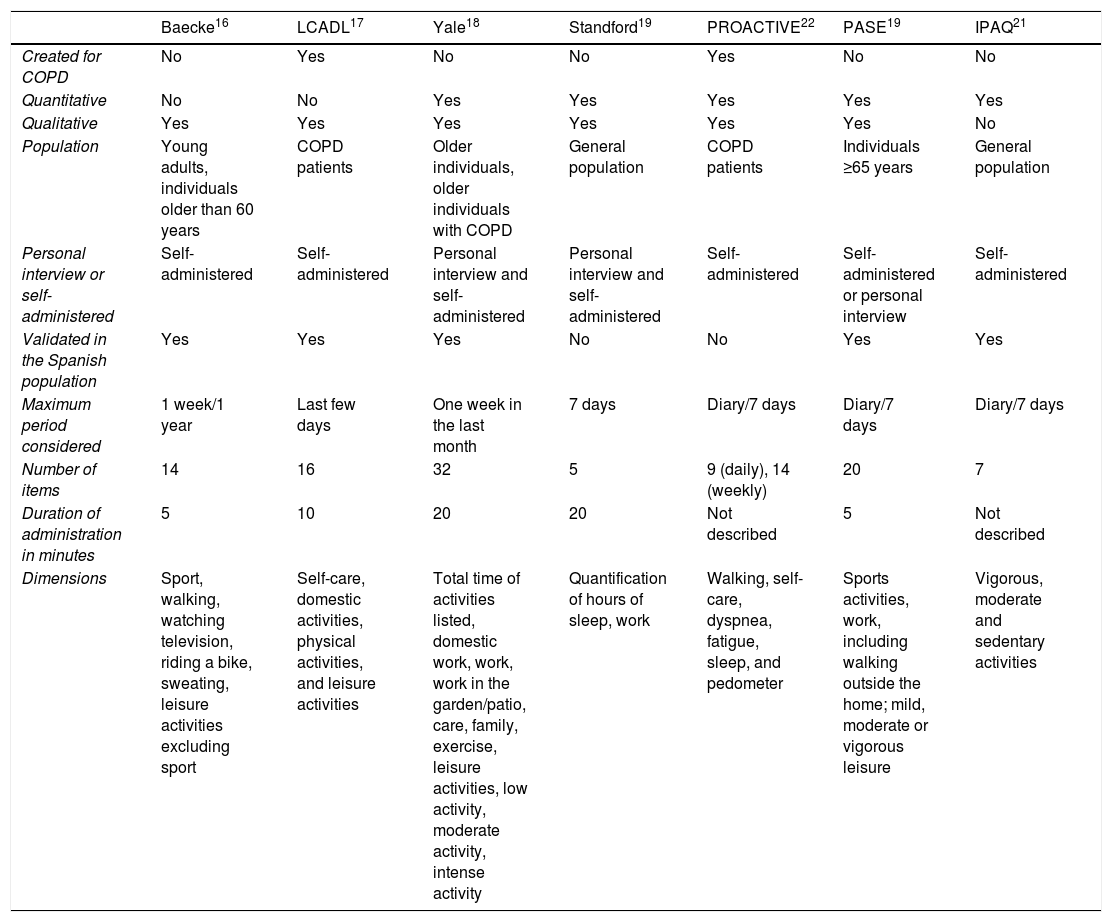
ResultsPhase 1: Review of Previous Physical Activity Questionnaires and Identification of Unmet NeedsThe following questionnaires were selected: Baeck,16 LCADL (London Chest Activity of Daily Living scale),17 Yale,18 Stanford Seven-Day Physical Activity Recall,19 PASE (Physical Activity Scale for the Elderly),20 IPAQ (Physical Activity Questionnaire Short-Form),21 and the questionnaire developed by the PROactive initiative22 (Table 1). Of these, only the PROactive22 and LCADL17 were developed specifically for COPD, although the Yale questionnaire18 was also validated for these patients at a later date.
Review of Physical Activity Questionnaires.
| Baecke16 | LCADL17 | Yale18 | Standford19 | PROACTIVE22 | PASE19 | IPAQ21 | |
|---|---|---|---|---|---|---|---|
| Created for COPD | No | Yes | No | No | Yes | No | No |
| Quantitative | No | No | Yes | Yes | Yes | Yes | Yes |
| Qualitative | Yes | Yes | Yes | Yes | Yes | Yes | No |
| Population | Young adults, individuals older than 60 years | COPD patients | Older individuals, older individuals with COPD | General population | COPD patients | Individuals ≥65 years | General population |
| Personal interview or self-administered | Self-administered | Self-administered | Personal interview and self-administered | Personal interview and self-administered | Self-administered | Self-administered or personal interview | Self-administered |
| Validated in the Spanish population | Yes | Yes | Yes | No | No | Yes | Yes |
| Maximum period considered | 1 week/1 year | Last few days | One week in the last month | 7 days | Diary/7 days | Diary/7 days | Diary/7 days |
| Number of items | 14 | 16 | 32 | 5 | 9 (daily), 14 (weekly) | 20 | 7 |
| Duration of administration in minutes | 5 | 10 | 20 | 20 | Not described | 5 | Not described |
| Dimensions | Sport, walking, watching television, riding a bike, sweating, leisure activities excluding sport | Self-care, domestic activities, physical activities, and leisure activities | Total time of activities listed, domestic work, work, work in the garden/patio, care, family, exercise, leisure activities, low activity, moderate activity, intense activity | Quantification of hours of sleep, work | Walking, self-care, dyspnea, fatigue, sleep, and pedometer | Sports activities, work, including walking outside the home; mild, moderate or vigorous leisure | Vigorous, moderate and sedentary activities |
There are many different methods of measuring the duration, intensity and frequency of PA, and not all specifically measure inactivity. Mean duration for completion is over 10min, which is excessively long. It was agreed unanimously that none of the referenced questionnaires was routinely used in daily clinical practice. The PA described in the items involved relatively demanding activities, with very little discriminative capacity, particularly among patients with COPD with less functional involvement. The reviewed questionnaires do not assess inactivity or sensitivity to change, nor do they study the factors determining low PA in any depth. The conclusion was that a simple questionnaire that could be used in clinical practice was required.
Phase 2: Identification of Dimensions and Questionnaire ItemsA total of 148 items were reviewed, of which 3 were rated “very much agree” by 83.3% of panel members and thus selected directly; 2 of these were taken from the PROactive22 instrument (“How much walking did you do outside today?” and “In the past 7 days, how much walking did you do outside?”), and 1 from the PASE20 (“On average, how many hours per day did you spend walking?”). Another 45 criteria were selected after another round of opinion and discussion. The remaining criteria were discarded, and the first proposal for the questionnaire was drafted. Taking into account the review undertaken and the situation of the COPD patient, the questionnaire structure should include PA intensity (low, moderate, and intense), and inactivity or a sedentary lifestyle, as this is considered to complement PA itself. The amount and frequency of PA must also be measured. The panel members suggested that the questionnaire should offer the physician additional information on the patient with low activity and the impact of their limitations. Investigating the possible causes or motives of low PA could provide data that might form a useful basis for therapeutic intervention. This way, the impact would be defined as the patient's perception of their expectations of activity, which would include their personal experience, characteristics of their environment, and their personality.
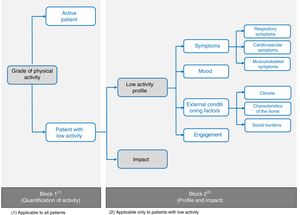
Phase 3: Structure of the SAQ-COPD QuestionnaireThe new questionnaire is divided into 2 blocks. The first is a tool aimed at quantifying PA and differentiating between active patients and those with low activity levels, and the second, which is informative in nature, will be completed only by patients with low PA, in order to define their profile and the impact of their limitations.
Block 1: Quantification of Physical ActivityWith regard to structure, 80% of panel members agreed that the tool should consist of quantitative and qualitative questions. However, after taking into account the level of information and the need to limit the number of questions, it was decided in subsequent discussion to include only quantitative questions. The panel agreed to remove the questions on self-care and domestic activities, as these were considered typical of more advanced disease, or gender discriminatory, respectively. Items that would be easily interpreted by the patients were selected.
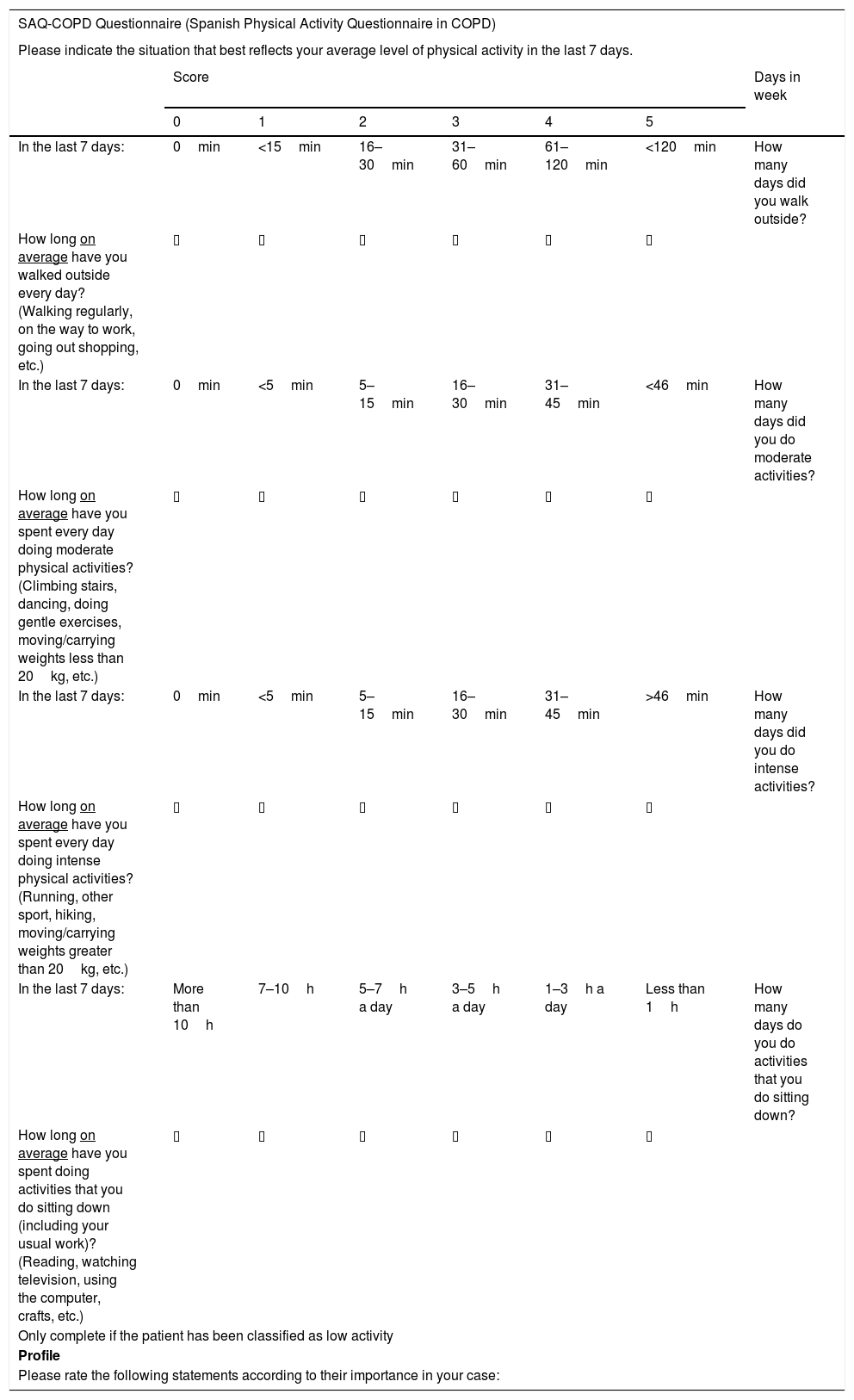
The previously mentioned questionnaires were used as a reference to define the level of PA, along with the list of examples given by the World Health Organization to define the term PA.23 The use of examples of activities with a similar metabolic expenditure was proposed, for which the commonly accepted MET (metabolic equivalent) intervals were used.2 Low PA was represented by the “walking” item, with examples such as regular, gentle walking, going out to work, and going shopping. Examples representing moderate PA included climbing stairs, dancing, doing gentle exercises or carrying/moving weights of less than 20kg, while intense activity was represented with examples such as running, doing sports, hiking, and carrying/moving weights of more than 20kg. Finally, it was agreed that inactivity would be described with examples that involve sitting down, reading, watching television, using the computer, or doing handicrafts (Table 2).
SAQ-COPD Questionnaire (Spanish Physical Activity Questionnaire in COPD).
| SAQ-COPD Questionnaire (Spanish Physical Activity Questionnaire in COPD) | |||||||
|---|---|---|---|---|---|---|---|
| Please indicate the situation that best reflects your average level of physical activity in the last 7 days. | |||||||
| Score | Days in week | ||||||
| 0 | 1 | 2 | 3 | 4 | 5 | ||
| In the last 7 days: | 0min | <15min | 16–30min | 31–60min | 61–120min | <120min | How many days did you walk outside? |
| How long on average have you walked outside every day? (Walking regularly, on the way to work, going out shopping, etc.) | ▯ | ▯ | ▯ | ▯ | ▯ | ▯ | |
| In the last 7 days: | 0min | <5min | 5–15min | 16–30min | 31–45min | <46min | How many days did you do moderate activities? |
| How long on average have you spent every day doing moderate physical activities? (Climbing stairs, dancing, doing gentle exercises, moving/carrying weights less than 20kg, etc.) | ▯ | ▯ | ▯ | ▯ | ▯ | ▯ | |
| In the last 7 days: | 0min | <5min | 5–15min | 16–30min | 31–45min | >46min | How many days did you do intense activities? |
| How long on average have you spent every day doing intense physical activities? (Running, other sport, hiking, moving/carrying weights greater than 20kg, etc.) | ▯ | ▯ | ▯ | ▯ | ▯ | ▯ | |
| In the last 7 days: | More than 10h | 7–10h | 5–7h a day | 3–5h a day | 1–3h a day | Less than 1h | How many days do you do activities that you do sitting down? |
| How long on average have you spent doing activities that you do sitting down (including your usual work)? (Reading, watching television, using the computer, crafts, etc.) | ▯ | ▯ | ▯ | ▯ | ▯ | ▯ | |
| Only complete if the patient has been classified as low activity | |||||||
| Profile | |||||||
| Please rate the following statements according to their importance in your case: | |||||||
| It doesn’t bother me at all | It completely inhibits me | |||||
|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | |
| I don’t do more physical activity mainly because I am short of breath (or I get fatigued) | ▯ | ▯ | ▯ | ▯ | ▯ | ▯ |
| I don’t do more physical activity mainly because my legs get tired | ▯ | ▯ | ▯ | ▯ | ▯ | ▯ |
| I don’t do more physical activity mainly because it hurts my back or my legs | ▯ | ▯ | ▯ | ▯ | ▯ | ▯ |
| I don’t do more physical activity mainly because I feel discouraged or I am afraid | ▯ | ▯ | ▯ | ▯ | ▯ | ▯ |
| I don’t do more physical activity mainly because I have nowhere to do it in my neighborhood or because I get too tired entering or leaving the building where I live | ▯ | ▯ | ▯ | ▯ | ▯ | ▯ |
| I don’t do more physical activity mainly because I have to take care of a family member | ▯ | ▯ | ▯ | ▯ | ▯ | ▯ |
| I don’t do more physical activity because I am discouraged by the weather where I live | ▯ | ▯ | ▯ | ▯ | ▯ | ▯ |
| I don’t do physical activity mainly because I am embarrassed | ▯ | ▯ | ▯ | ▯ | ▯ | ▯ |
| I don’t do physical activity because I don’t like exercising | ▯ | ▯ | ▯ | ▯ | ▯ | ▯ |
| Impact |
|---|
| To what extent do you agree about the impact of physical activity on your health and disease? Please rate the following statements according to your case: |
| Not at all | Totally | |||||
|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | |
| I believe that the lack of activity worsens my disease | ▯ | ▯ | ▯ | ▯ | ▯ | ▯ |
| The lack of activity prevents me from having more social life | ▯ | ▯ | ▯ | ▯ | ▯ | ▯ |
| I think that the lack of activity makes me more dependent on other people | ▯ | ▯ | ▯ | ▯ | ▯ | ▯ |
| I believe that if I did more activity I would be in a better mood/have more energy | ▯ | ▯ | ▯ | ▯ | ▯ | ▯ |
| When I compare myself with other people my age, I think my physical activity is similar to that of others | ▯ | ▯ | ▯ | ▯ | ▯ | ▯ |
| Thank you very much for your collaboration. |
| © Copyright 2017 AstraZeneca Farmacéutica Spain, S.A. All rights reserved. |
| Reproduction or dissemination of any part of this document in any format without the express written permission from the copyright holder is strictly forbidden. |
The structure of the questionnaire was designed with the aim of achieving good applicability and ease of use. The expert panel found in their standard clinical practice that the structure of the COPD Assessment Test24 is particularly easy to use and applicable in a care setting, so they agreed on the use of a similar structure with 6-point Likert scales25 for each of the blocks. In order to make the questionnaire easier for the patient to complete, a single question for each PA dimension was used, with a time frame of the last 7 days, and a question on frequency (average number of days per week) was added. The low activity block uses the following categories: 0=0min, 1≤15min, 2=16–30min, 3=31–60min, 4=61–120min and 5≥120min, while for moderate or intense exercise the time interval was reduced, as proposed in the Baecke questionnaire: 0=0min, 1=<5min, 2=5–15min, 3=16–30min, 4=31–45min and 5=46–60min. In the dimension of inactivity, the score is assigned in reverse: 0≥10h/day, 1=7 to 10h/day, 2=5–7h/day, 3=3–5h/day, 4=1–3h/day and 5≤1h/day. Completion of this first block of the SAQ-COPD will provide an overall score that will be used to classify the patient as active or low PA.
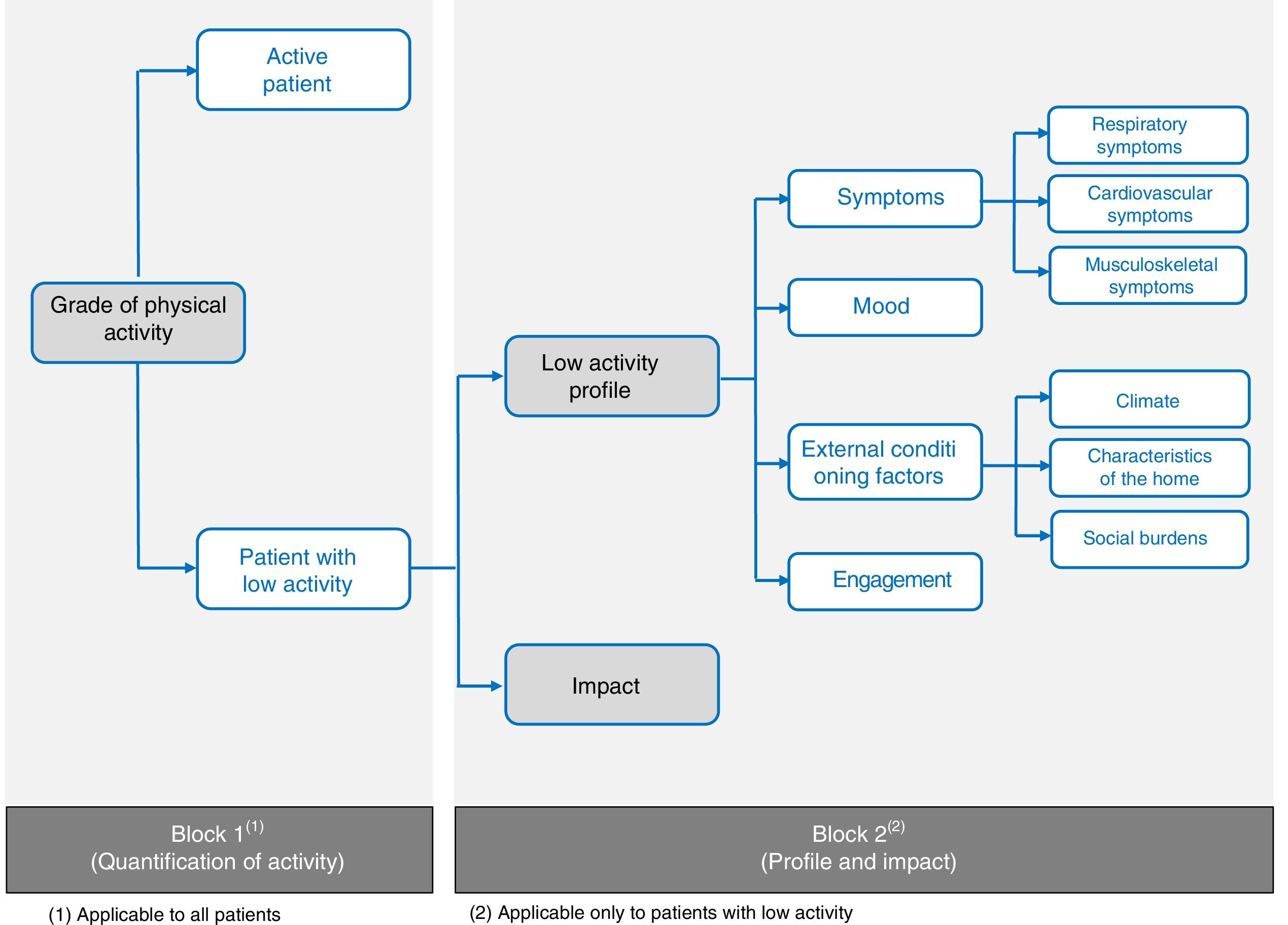
Block 2: Profile and ImpactThis second questionnaire block will only be completed by patients with low PA according to the result obtained in Block 1. Nine profile items were defined and divided into 4 patient profiles: (a) low PA according to symptoms (respiratory, musculoskeletal, and cardiocirculatory); (b) mood; (c) external conditions (climate, characteristics of the home, and social burden); and (d) engagement (Fig. 2).
Finally, to evaluate impact, items were created for comparing the patient with other individuals of the same age in the same environment, providing information about their expectations and whether their lack of PA made them more dependent. Thus, the first version of the SAQ-COPD questionnaire was drafted (Table 2).
Linguistic ComprehensionSixteen patients were included with a mean age of 64.6 years; 85% men, 12.5% with no education, 56.25% with primary education, 25% with secondary education, and 6.25% with university education; 20% unemployed and 80% retired. Diagnosis was severe COPD in 75%, moderate COPD in 18.75%, and mild COPD in 6.25%. Total mean time for questionnaire completion was 4.53min (63.57s for the activity dimension, 142.3s for the patient profile, and 66.28s for impact).
Results showed that over 30% of the participants had difficulties understanding the word “mean” (promedio in Spanish), so it was changed to “average” (por termino medio in Spanish). Another 3 items were reworded: “I do not do more physical activity principally because I have nowhere to do it in my neighborhood or because I have to make a major effort to get in or out of the house” was changed to “I do not do more physical activity mainly because I have nowhere to do it in my neighborhood or because I get too tired entering or leaving the building where I live”; “I do not do more physical activity principally because I have to take care of a family member” was changed to “I do not do more physical activity mainly because I have to take care of a family member”; and “I do not do physical activity principally because I am embarrassed” was changed to “I do not do physical activity mainly because I am embarrassed”. These changes were verified in 14 patients with mean age of 65.14 years; 92.8% men; 21.4% with no education, 64.2% with primary education and 14.2% with secondary education; 85.7% retirees; 57.1% with moderate COPD and 42.8% with severe COPD. The results confirmed perfect comprehension of the modified items.
DiscussionThe new physical activity questionnaire for COPD patients (SAQ-COPD) has several new features: (1) it is a simple, easy-to-complete questionnaire, which in our opinion can be used in clinical practice; (2) it is specifically designed for patients with COPD of any intensity; and (3) it has the additional advantage of investigating the reasons or motives that induce physical inactivity (low PA profile) and the impact of inactivity on the patient. All these potential benefits must be verified and validated in a future study that will refine this preliminary version.
While developing the questionnaire, priority was given to ease of administration and the discriminatory capacity of the tool. With regard to ease of administration, the block design and the clarity of the questions should ensure that the instrument is quick to complete, making it appropriate for administration by professionals in routine clinical practice. The results of the cognitive validation have shown that the questionnaire is simple (takes less than 5min to complete) and easily understood by patients.
Information collected on the amount, intensity, and frequency of activity and inactivity, along with a careful selection of items and examples, must be evaluated to confirm the instrument's capacity to discriminate PA limitations even in the early stages of the disease, or to analyze changes over time. Other studies have attempted to compare PA evaluated in questionnaires with objective motion sensors, but results have been varied.19,26 Recently, the utility of 4 questionnaires has been compared with PA measured by accelerometers in COPD patients.19 Unfortunately, correlation between the objective measurement of PA and the questionnaire score was poor in all cases. The authors recognize that this issue must be specifically addressed in the validation study, and scoring thresholds will be set that will better distinguish between low and adequate PA at different levels of disease severity. Sensitivity to change must also be an objective of the future validation.
As mentioned above, COPD patients sometimes have sufficient functional capacity to perform certain activities, but do not do so for different reasons (concomitant diseases, deliberate behavior, physical or functional impediment). However, this issue is not addressed by current PA questionnaires.16–22 Therefore, the aim of this questionnaire is not only to detect a decrease in PA, but also to define why the patient's PA level is reduced, since there may be underlying reasons that could be susceptible to therapeutic intervention. This approach is used in other aspects of the same disease, such as the Test of Adherence to Inhalers, which has the added value of classifying the causes of non-adherence.27 Similarly, this questionnaire must be able to classify patients according to their low activity profile.
The panel also underlined the importance of the patient's own engagement and their expectations with regard to with their current PA status, factors which should be assessed at the time of planning an intervention. Thus, the concept of impact was defined and included in the questionnaire. This measurement is designed to show if the limitation is endured or accepted by the patient, in which case he/she may not be receptive to a therapeutic action.
In conclusion, in this article we present to the scientific community the SAQ-COPD questionnaire, a tool with high potential in terms of applicability and sensitivity. However, the subsequent validation procedure in COPD patient may generate changes in the form and content of the questionnaire. The validation process began with the evaluation of the comprehension and cognitive validity of the tool in a small group of COPD patients, and will later include the remaining psychometric properties (feasibility analysis, floor and ceiling effects, concept validity, criterion validity vs accelerometer, construct validity, internal consistency, test-retest reliability, and sensitivity to change) in a wider analysis in the same type of patients. The validation applies specifically to the first block of the questionnaire, which discriminates if the patient is active or presents low PA. Given the complexity of validating the causes that underlie the patient's profile, this information will not undergo the validation process, although it will be analyzed. Finally, the validation of the impact of a low PA compared to the COPD Assessment Test is planned.
FundingThis study was funded by an unrestricted grant from AstraZeneca Farmaceutica Spain, S.A.
Conflicts of InterestJuan José Soler Cataluña has received fees for scientific consultancy or for speaking engagements from AstraZeneca, Boehringer Ingelheim, Chiesi, Ferrer, GlaxoSmithKline, Laboratorios Esteve, Menarini, Mundipharma, Novartis, Rovi and Teva.
Luis Puente Maestu has received payment for consultancy, research grants and funds for courses from AstraZeneca, Boehringer Ingelheim, Boston Scientific, Chiesi, Esteve, GSK, Menarini, Novartis, Roche, Vyasis and Zambon.
Miguel Román-Rodríguez has received fees for scientific consultancy or for speaking engagements from AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Menarini, Mundipharma, Novartis, Pfeizer, Rovi and Teva.
Cristóbal Esteban has no conflicto of interest with regard to this article.
Joaquín Gea has no conflicto of interest with regard to this article.
Roberto Bernabeu Mora has received fees for scientific consultancy or for speaking engagements from AstraZeneca, Menarini, Boehringer Ingelheim, Esteve, Novartis, Ferrer, Rovi, Pfizer, Orion, Chiesi, Teva, GlaxoSmithKline and Mundipharma.
Eulogio Pleguezuelos Cobo has no conflicto of interest with regard to this article.
Gema Montegaudo Ruiz is currently an employee of AstraZeneca S.A.
Julio Ancochea has received fees for scientific consultancy or for speaking engagements from Actelion, Air Liquide, Almirall, AstraZeneca, Boehringer Ingelheim, Carburos Médica, Chiesi, Faes Farma, Ferrer, GlaxoSmithKline, InterMune, Linde Healthcare, Menarini, MSD, Mundipharma, Novartis, Pfizer, Roche, Rovi, Sandoz, Takeda and Teva.
Francisco García Rio has received funding for research projects, scientific consultancy and conferences from AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Esteve, Novartis and Teva.
The authors gratefully acknowledge the editorial assistance of Antonio Torres-Ruiz (Dynamic Science S.L.). The opinions, interpretation of data, and conclusions contained in this article are the responsibility of the authors.
Please cite this article as: Soler-Cataluña JJ, Maestu LP, Román-Rodríguez M, Esteban C, Gea J, Mora RB, et al. Creación del cuestionario SAQ-COPD (Spanish Physical Activity Questionnaire in COPD) para la medida de la actividad física de pacientes con EPOC en la práctica clínica. Arch Bronconeumol. 2018;54:467–475.