Lung ultrasound (LUS) is currently an indispensable tool in pulmonology. It has great advantages, including reproducibility, low cost, and lack of ionizing radiation, but its main limitations are its incompatibility with air, and the fact that it is operator-dependent. LUS findings in healthy lung parenchyma generate various signs, such as the pleural shift sign, and artifacts called A and B lines.
Specifically, more than 3 B lines appearing in a scanned area can be interpreted as thickening of the interlobular septa. Roughness, thickening and destructuring of the pleural line, in addition, may be a sign of interstitial lung disease (ILD).1–4
To date, no ultrasound method has been able to quantify the severity of lung parenchyma involvement in patients with diffuse ILD, an application that might also be useful in follow-up. For this reason, high-resolution computed tomography (HRCT) continues to be essential for evaluating of the severity and progress of these patients.5–8
The most commonly used ultrasound technique for assessing fibrosis (more specifically, liver fibrosis) is ultrasound elastography, although conventional ultrasonography, based on morphological grayscale analysis, may contain more information than that provided by elastography. The acoustic structure quantification (ASQ) method is a non-invasive tool that is used to characterize tissues through the statistical analysis of ultrasound signals received. When tissue is normal, the echo pulse generated is smaller than the ultrasound wavelength, following the normal Rayleigh distribution (continuous distribution function). When tissue is fibrotic, the echo pulses become larger than the wavelength, and deviate from the Rayleigh distribution.9–11
ASQ is currently used for characterizing liver fibrosis. Unlike elastography, ASQ images are produced by ultrasound interference generated by innumerable reflective objects. According to this theory, tissue heterogeneity could be quantified by measuring the speckle pattern of tissue from an analysis of the probability density function. On the basis of these principles, and given that no studies have been previously published, we studied ASQ as a method for the evaluation of diffuse ILD.12–15
We thus performed the first prospective, observational, randomized case-control study to determine if the ASQ method could quantify severity in fibrosing diffuse ILD. Two groups of patients were recruited, after obtaining ethics committee approval and written informed consent. Group 1 comprised patients with ILD and involvement demonstrated on high-resolution computed tomography (HRCT). Group 2 were healthy patients without respiratory disease, no history of smoking, and normal results on lung auscultation, spirometry, and chest X-ray.
The study was conducted in the pulmonology clinic, where both patient groups underwent evaluation, first with ultrasound and then with the ASQ method. We used a convex transducer, and multiple images were captured in B-mode applied between the intercostal spaces. In patients with diffuse ILD, the point with most interstitial involvement previously visualized on HRCT was sought. In healthy patients, the best axial plane of the lung bases was used.
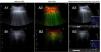
The acquired images were interpreted with ASQ analysis, drawing 3 randomly assigned regions of interest (ROI), which included the intercostal muscles, pleural line, and lung parenchyma. Mean and standard deviation were calculated for each measurement (data derived directly from the ultrasound equipment). In addition, a multiparametric map based on echo amplitude distribution was generated, in which high Cm2 values (a statistical parameter derived from the varying echo amplitude distribution) are represented in a darker gray and low values in a lighter gray. In liver fibrosis, dispersion increases proportionally with the distortion of the parenchymal architecture and produces a red color, so we applied this same premise in this study, but associating it with pulmonary fibrosis.
The values of heterogeneity of data obtained in the ROI were collected as mean and standard deviation, and comparisons among groups were performed using the Mann–Whitney test. Probability values were considered significant at P<0.05.
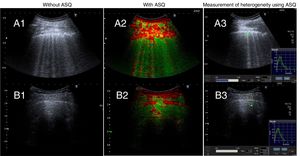
The study included a total of 20 patients (10 per group). In group 1, mean age was 74.7±8.8 years, and 60% were men. Pulmonary interstitial patterns with fibrotic component on HRCT were distributed as follows: usual interstitial pneumonia in 5 patients, non-specific interstitial pneumonia in 3, and chronic hypersensitivity pneumonitis in 2. LUS without ASQ (Fig. 1A) showed roughness, thickening and destructuring of the pleural line and multiple B lines (more than 3) per field explored. LUS with ASQ (Fig. 1A2 and A3) was represented with a multiparametric map, measuring heterogeneity in the pulmonary parenchyma (1.42±0.086), pleural line (1.58±0.172), and extrapleural line (1.16±0.138). In group 2, mean age was 44.1±5.8 years, and 30% were men. LUS without ASQ 1B1) showed a normal, fine pleural line, B lines (less than 3) and A lines. LUS with ASQ (Fig. 1A2 and A3) showed the multiparametric map, and measured the heterogeneity ratio in the pulmonary parenchyma (1.05±0.118), pleural line (1.43±0.178), and extrapleural line (1.32±0.150).
Pulmonary ultrasound without ASQ and with ASQ. Group 1. A1: without ASQ, showing roughness, thickening, and destructuring of the pleural line and multiple B lines. A2 and A3: with ASQ, multiparametric representation and measurement of lung heterogeneity, respectively. Group 2. B1: without ASQ, showing normal, fine pleural line, B lines (less than 3) and A lines. B2 and B3: with ASQ, multiparametric representation and measurement of lung heterogeneity, respectively.
Statistically significant differences were observed in the association between the quantification of tissue heterogeneity in pulmonary parenchyma (P<0.01) and extrapleural tissue (P<0.05) between both groups.
Our study is limited by its small sample size. However, given the findings of this first preliminary study, we conclude that LUS with ASQ could quantify the degree of interstitial involvement and guide the management of these patients. However, a study with a larger sample size and reproduction of the measurements obtained by ultrasound will be necessary.
FundingThe current manuscript had no source of funding.
Conflict of InterestsThe authors declare that they have no conflict of interests directly or indirectly related with the contents of this manuscript.
We thank everyone who collaborated in this study.
Please cite this article as: Wangüemert Pérez AL, González Delgado C, Fernández Ramos J. Aplicación ecográfica con cuantificación de la estructura acústica (ASQ) en las enfermedades pulmonares intersticiales. Arch Bronconeumol. 2019;55::539–540.