The diagnosis of peripheral pulmonary lesions (PPL) is important, especially when it is required to confirm or exclude malignancy. We present what we believe to be the first two cases of PPL diagnosed by transbronchial cryobiopsies guided by real-time transthoracic ultrasound and assisted by radial endobronchial ultrasound (r-EBUS).
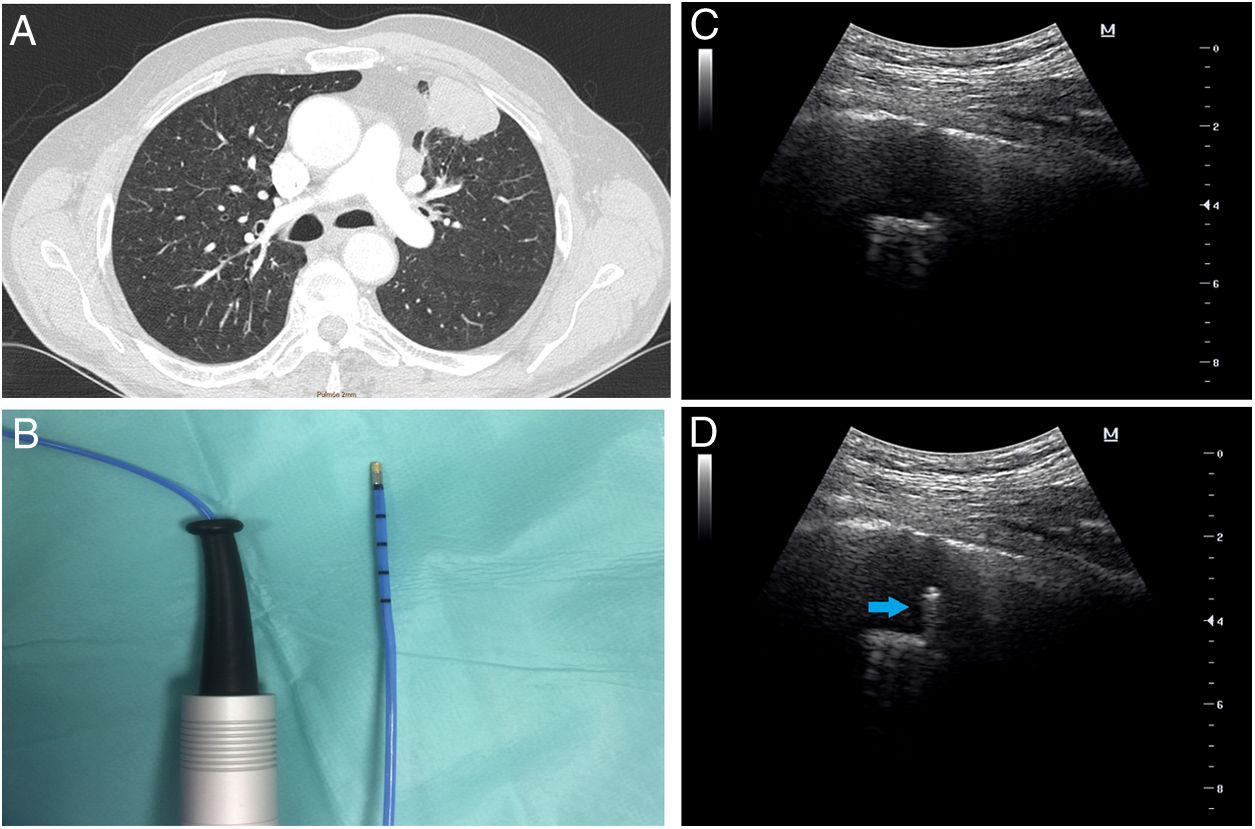
The first patient was a 76-year-old male, a heavy ex-smoker with a peripheral left mass on chest X-ray. Thoracic computed tomography (CT) revealed a lingular solid mass with 38mm larger diameter, based on pleura adjacent to the anterior thoracic wall (Fig. 1A) compatible with primary lung neoplasia. Chest ultrasound examination showed a nodule of 26mm of maximum depth with pleural contact, mobile with respiratory movements and partially covered by the adjacent rib, leaving a narrow intercostal space for examination. Standard bronchoscopy with a thin bronchoscope, showed no endobronchial lesions so the endoscopic examination was carried out with r-EBUS. A 1.4mm radial ultrasound probe (Olympus Co, Japan) was advanced through the lingula bronchi until the hypoechoic heterogeneous nodule was visualized and then we tried to reproduce the path with the 1.9mm cryoprobe (ERBE CA, Germany) (Fig. 1B). Prior to transbronchial sampling, the transthoracic ultrasound (convex transducer, F2.5MHz D9.9, MINDRAY Co, China) was applied on the anterior chest wall (Fig. 1C) and was able to visualize, in real time, the transbronchial cryoprobe going through the mass and avoiding the pleura (Fig. 1D and supplementary video 1A). Curiously, applying freezing decreased the ultrasound image of the probe, except for the tip (supplementary video 1B), and allowed for the visualization of the tissue freezing process. Biopsies (4 samples, 1.1×0.9×0.3cm) were positive for adenocarcinoma.
(A) Chest CT image shows a lingular mass with pleural contact and bronchus sign. (B) Flexible cryoprobe indicated for transbronchial biopsy. Outer diameter 1.9mm, length 900mm. (C) Transthoracic ultrasound image before performing transbronchial biopsy of the mass. (D) Transthoracic ultrasound image during the performance of transbronchial biopsy, which locates the cryoprobe within the lung mass (arrow).
The second case was a 39-year-old female who presented with fever and unresolved pneumonia which had resisted antibiotic therapy for a period of two months. The patient had no history of smoking and her physical examination revealed only bilateral basal fine crepitations. Thoracic CT showed bilateral basal consolidation opacities with positive air bronchogram. Microbiological examination for sputum and bronchoalveolar lavage samples were negative, including the tuberculosis work-up. For the diagnosis of the underlying pathology, the patient was subjected to r-EBUS assisted transbronchial cryobiopsy guided with transthoracic ultrasound. During the procedure, the radial probe was advanced through the lateral segment of the left lower lobe until the lesion was visible. Then, the 1.9mm flexible cryoprobe was advanced to the target location. Simultaneously, the transthoracic ultrasound was applied on the chest wall and was able to detect the lesion. The transthoracic ultrasound was helpful in confirming that the cryoprobe was located within the lesion and distant from the pleura to avoid pneumothorax. Four transbronchial cryobiopsies were obtained and the histopathological examination revealed cryptogenic organizing pneumonia.
Conventional bronchoscopy has a low diagnostic value for PPL which may be less than 20% for nodules smaller than 2cm in diameter.1 Also, it should be noted that not all PPL can be seen by fluoroscopy, in addition to the presence of radiation hazards for the patient and the operators. Although CT guided percutaneous biopsy is a reliable technique for the diagnosis of these lesions, it is associated with a high risk of pneumothorax2 and is therefore usually indicated when transbronchial procedures have proved unsuccessful. Chest ultrasound guided percutaneous biopsy is an equally long-established method but requires appropriate case selection in advance.3 The pleural contact length made by the mass or nodule, for example, influences diagnostic yield. Chest ultrasound and CT guided percutaneous biopsy were not considered a convenient alternative because of the ultrasound findings in our first case and because of diagnostic suspicion in the second one. Moreover, these techniques do not allow for the obtention of cryobiopsy samples.
Recent updates to bronchoscopic methods have aimed to increase the diagnostic value for PPL. Electromagnetic navigation bronchoscopy has achieved an accuracy of up to 81.7%, however, it is associated with high economic costs.4 R-EBUS has emerged as a safer method in comparison to CT-guided biopsy but the yield of r-EBUS remains lower than that of CT-guided biopsy, due in part to the smaller sample size.5,6
The safety and feasibility of cryoprobes in combination with r-EBUS has also undergone a preliminary evaluation for the diagnosis of PPL and compared with forceps biopsies. Transbronchial cryobiopsy with r-EBUS assistance can obtain a better diagnostic yield and significantly larger samples.7,8 Cryobiopsy could even increase the detection rate of epidermal growth factor receptor (EGFR) mutations in non-small-cell lung cancer in comparison to other tissue sampling techniques.9 However, a meta-analysis10 reported a pneumothorax average incidence of 10% after transbronchial biopsies with a cryoprobe in interstitial lung disease. Bleeding during cryobiopsy is common and the risk is greater than with conventional forceps biopsies. Moderate bleeding after cryo-biopsy was observed in 65 cases of 383 patients in 12 studies (16.9%).10
A main drawback regarding r-EBUS is the absence of real-time vision for biopsy. In this case report, we describe a novel technique for the biopsy of malignant and benign PPL near to pleura using transthoracic ultrasound guidance for cryobiopsies. The particular advantages of real-time guidance with external thoracic ultrasound are the ability to guide the biopsy tool directly to the lesion without C-arm fluoroscope, using echotexture to avoid necrotic areas, preventing injury to surrounding vascular structures and the pleura and to permit the exclusion of the pneumothorax.11 For instance, in the diagnosis of interstitial lung diseases, distances of the cryoprobe to the pleura of less than 1cm are associated with a significantly increased risk of pneumothorax,12 while biopsies obtained too proximally to the middle third of the lung without ultrasound assistance increase the risk of severe bleeding.
Also, we hypothesize that guided real-time transthoracic ultrasound can increase the diagnostic yield of r-EBUS in a way that is similar to real-time convex EBUS transbronchial needle aspiration13 when compared with non-real-time EBUS.14 Nevertheless, in comparison with CT-guided biopsy, our technique may have the disadvantage of requiring two operators, one for bronchoscopy and the other for performing transthoracic ultrasound. Even though, thoracic ultrasound offers other advantages, including the lack of ionization radiation, low cost, flexibility, reproducibility of the examination and bedside availability.15
In conclusion, the combination of transthoracic ultrasound and flexible cryoprobe add to r-EBUS procedure the potentiality of vision-guided transbronchial cryobiopsies for PPL lesions with pleural contact. Using percutaneous chest ultrasound has enabled us to guide the cryoprobe to the target lesion to obtain accurate samples. Further benefits are visual confirmation that it is freezing the tissue, visualization of the distance to the pleura to prevent pneumothorax and the use of the color Doppler to avoid highly vascularized areas.
Our initial experience requires prospective validation in a larger patient cohort to confirm the reliability and reproducibility of these results combining transbronchial cryobiopsy with percutaneous chest ultrasound.
FundingThis study was partially supported by grants AEER 2016, SEPAR 120/2016 and FUCAP 2017.
Conflict of interestThe authors have reported to Archivos de Bronconeumología that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
The following are the supplementary data to this article: