The objective of this study was to assess tracheal reactivity after the deployment of different self-expandable metal stents (SEMS).
Material and methodsForty female New Zealand rabbits were divided into four groups. Three groups received three different SEMS: steel (ST), nitinol (NiTi), or nitinol drug-eluting stent (DES); the fourth group was the control group (no stent).
Stents were deployed percutaneously under fluoroscopic guidance. Animals were assessed by multi-slice, computed tomography (CT) scans, and tracheas were collected for anatomical pathology (AP) study. Data from CT and AP were statistically analyzed and correlated.
ResultsThe DES group had the longest stenosis (20.51±14.08mm vs 5.84±12.43 and 6.57±6.54mm in NiTi and ST, respectively, day 30; P<.05), and higher granuloma formation on CT (50% of cases). The NiTi group showed the lowest grade of stenosis (2.86±6.91% vs 11.28±13.98 and 15.54±25.95% in DES and ST, respectively; P<.05).
The AP study revealed that the ST group developed intense proliferative reactivity compared to the other groups. In the DES group, a destructive response was observed in 70% of the animals, while the NiTi was the least reactive stent.
CT was more effective in detecting wall thickening (positive correlation of 68.9%; P<.001) than granuloma (not significant).
ConclusionsThe ST group developed granulomas and significant stenosis. NiTi was the least reactive stent, while DES caused significant lesions that may be related to drug dosage. This type of DES stent is therefore not recommended for the treatment of tracheobronchial stenosis.
El objetivo de este estudio es evaluar la reactividad traqueal tras la implantación de distintos stents metálicos autoexpandibles (SMAE).
Material y métodosSe utilizaron 40 conejos hembra de raza neozelandesa, que se dividieron en 4 grupos. En 3 grupos se implantaron SMAE: de acero (SA), de nitinol (NiTi) o stents liberadores de nitinol (SLF). El cuarto grupo fue el grupo de control (sin stent).
Los stents se implantaron por vía percutánea bajo control fluoroscópico. Los animales se evaluaron mediante tomografía axial computarizada (TAC) multicorte y las tráqueas se extirparon para su estudio anatomopatológico (EAP). Los datos de la TAC y el EAP se analizaron estadísticamente y se correlacionaron.
ResultadosEl grupo que recibió SLF presentaba la mayor longitud de estenosis (20,51±14,08mm frente a 5,84±12,43 y 6,57±6,54mm en los grupos NiTi y SA, día 30; p<0,05) y el mayor índice de formación de granulomas evidenciados mediante TAC (50% de los casos). El grupo al que se implantaron stents NiTi mostró el menor grado de estenosis (2,86±6,91% frente a 11,28±13,98 y 15,54±25,95% en los grupos SLF y SA; p<0,05).
En el estudio AP, el grupo SA presentó reactividad proliferativa intensa en comparación con los otros 2 grupos. En el grupo SLF se observó una respuesta destructiva en el 70% de animales, mientras que el stent NiTi fue el que menos reacción provocó.
La TAC resultó ser superior para detectar el engrosamiento (correlación positiva de un 68,9%; p< 0,001) que para la observación de granulomas (n.s.).
ConclusionesEl grupo SA desarrolló granulomas y estenosis significativas. El stent NiTi fue el que menos reacción indujo, mientras que el SLN provocó lesiones importantes que podrían estar relacionadas con la dosis de fármaco. Por consiguiente, este tipo de SLF no se recomienda para el tratamiento de la estenosis traqueobronquial.
Although surgical resection is the primary procedure for the treatment of tracheobronchial stenosis,1 tracheal stenting, along with other minimally invasive techniques, such as radiofrequency, laser ablation, and cryotherapy, provides satisfactory management of central airway obstruction in non-surgical patients.2 Silicone prostheses are the most commonly used, but these require the use of a rigid bronchoscope with general anesthesia for implantation. In contrast, metal stents can be deployed using fluoroscopy or through a flexible fiberoptic bronchoscope or endotracheal tube under light sedation, providing immediate symptomatic relief.3 Other advantages of metal stents over silicone models are their higher radial force, better internal to external diameter ratio, lower incidence of migration, and decreased risk of mucus obstruction and bacterial colonization.4 However, these important advantages have been clouded by long-term complications (such as restenosis due to granuloma formation or tumor growth), and difficulties involved in their removal.5 These issues led the Food and Drug Administration (FDA) in 2005 to advise against the use of metal stents in benign lesions.6
Nevertheless, since then, studies have reported that metal stents are safe for the treatment of benign and malignant tracheobronchial stenosis.6–9 Restenosis due to intraluminal overgrowth has been described mainly for steel stents.10 Studies with laser cut, self-expandable nitinol stents have had better results. However, these nitinol stents are mostly used for other territories such as cardiovascular and biliary tree indications. Antiproliferative drugs are used in cardiovascular indications to avoid restenosis,11,12 but experience in airway disease is limited.
We hypothesized that DES may combine the advantages of the metal stents in tracheal stenosis management, while avoiding or attenuating restenosis from intraluminal overgrowth through the mesh and over the ends. Therefore, the purpose of this study was to assess the tracheal responses to three different SEMS (drug-eluting nitinol, nitinol, and stainless steel bare metal stents) in an animal model.
MethodsAnimals and StentsForty adult female New Zealand rabbits (3.95±0.48kg) were used in this study. The care and use of animals complied with the European Communities Council Directive (86/609/EEC) and local animal welfare laws, guidelines and policies, and was approved by the University of Zaragoza ethics committee. Animals were randomly distributed into four groups: ST (n=10), steel stent (Wallstent™, Boston Scientific, Natick, MA. USA); NiTi (n=10), nitinol stent (Zilver®Flex™ Vascular Stent, Cook Medical, Bjaeverskov, Denmark); DES (n=10), paclitaxel-eluting nitinol stent (Zilver®PTX® Drug Eluting Peripheral Stent, Cook Medical, Bjaeverskov, Denmark); and control (n=10), with no stent. Both nitinol stents are laser-cut and have exactly the same pattern design, whereas the steel stent is braided wire. All stents were self-expandable and measured 8mm×40mm, at a 1:1 ratio to the trachea of the animal model. The stents were deployed percutaneously under general anesthesia and fluoroscopic guidance. Animals were followed for 90 days.
Stenting TechniqueBefore stent implantation, all rabbits were examined to ensure that they were healthy, and fasted for 8h. They were medicated intramuscularly with 0.5mg/kg medetomidine (Sedator®, Eurovet Animal Health, Netherlands) and 25mg/kg ketamine (Imalgene 1000®, Merial, Barcelona, Spain). Anesthesia was maintained with 1%–2% isofluorane (Isovet, Braun, Barcelona, Spain) by inhalation. Animals were monitored throughout the procedure (Samurai anesthetic equipment, La Bouvet, Madrid, Spain and Dash 3000 monitor, General Electric Company, Helsinki, Finland).
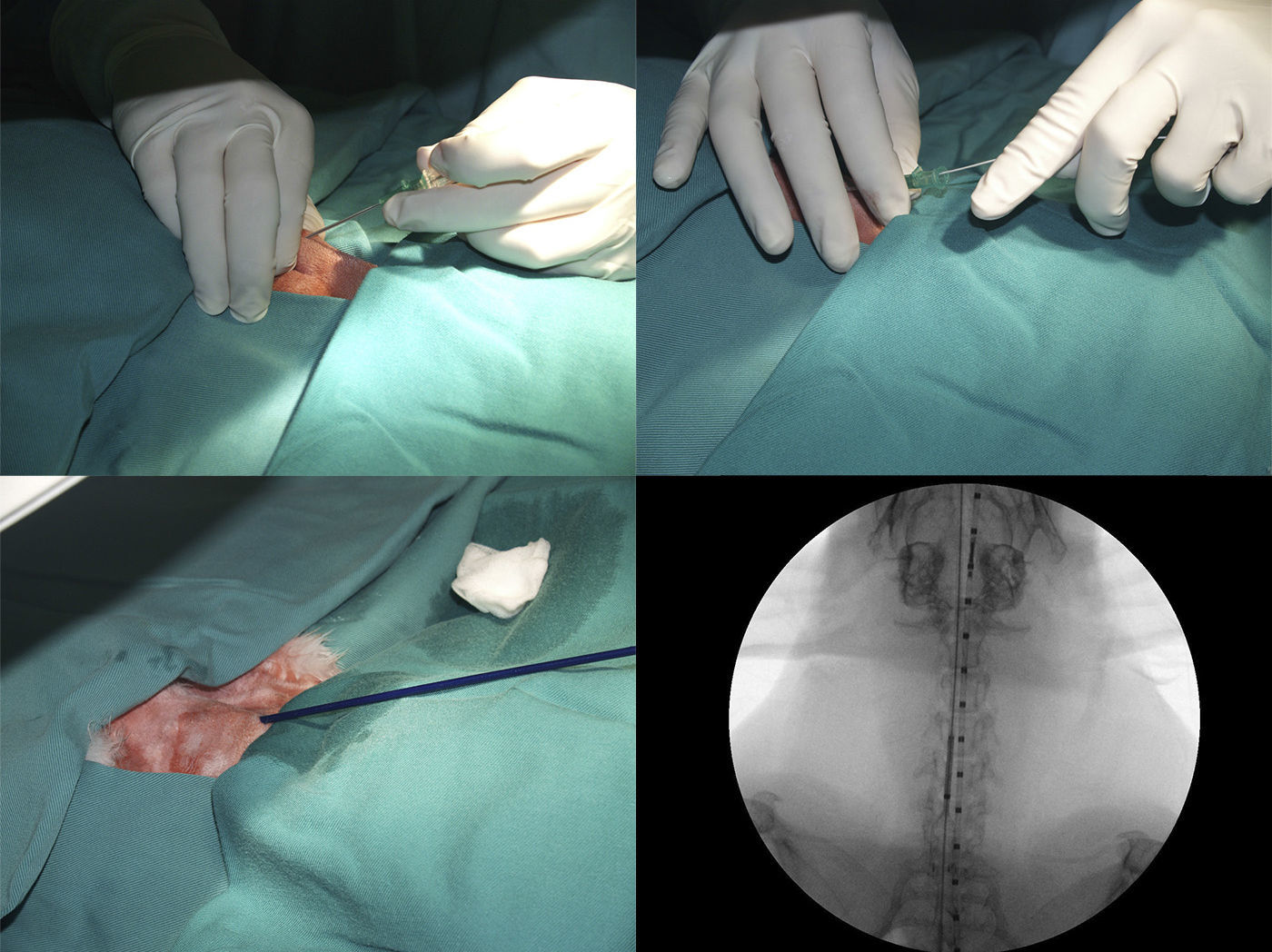
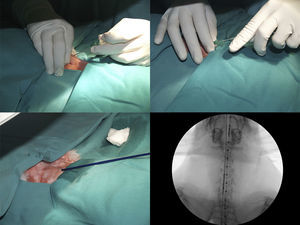
After positioning animals in supine recumbency with neck hyperextension, and administering 50mg/kg of oxytetracycline (Terramicina LA, Pfizer, Madrid, Spain), a straight 5F centimeter sizing catheter (Aurous®, Cook Medical, Bjaeverskov, Denmark) was introduced into the esophagus to obtain a reference measurement. Tracheal access was gained by puncturing between the two most cranial tracheal rings using an 18G catheter-over-needle (Introcan®, Braun, Germany), then 0.15ml lidocaine (Braun, Barcelona, Spain), was introduced into the trachea. A 0.035inch hydrophilic guide wire (Radifocus® Guide Wire M Standard type, Terumo, Leuven, Belgium), soaked in lidocaine, was advanced into the trachea, and the catheter was removed. The stent delivery system was inserted directly over the guide wire and placed at a defined position (distal mark of the stent, 1cm cranial to the tracheal carina) where the stent was deployed under fluoroscopic guidance (C-arm system BV Endura, Philips, Eindhoven, Netherlands). Once placed, the delivery system, guide wire, and centimeter catheter were removed, and the animal was supervised until recovery (Fig. 1).
All procedures were performed in the same way for the control group, using a stent delivery system without a stent.
Follow-Up and CT StudyAfter stent placement, all animals were observed for any sign of respiratory tract obstruction and surgical wound infection. The general health status of the animals was assessed by daily clinical examinations.
In cases of death before the end of the study, samples were obtained and processed immediately as per protocol. The only test that could not be performed after death was CT imaging.
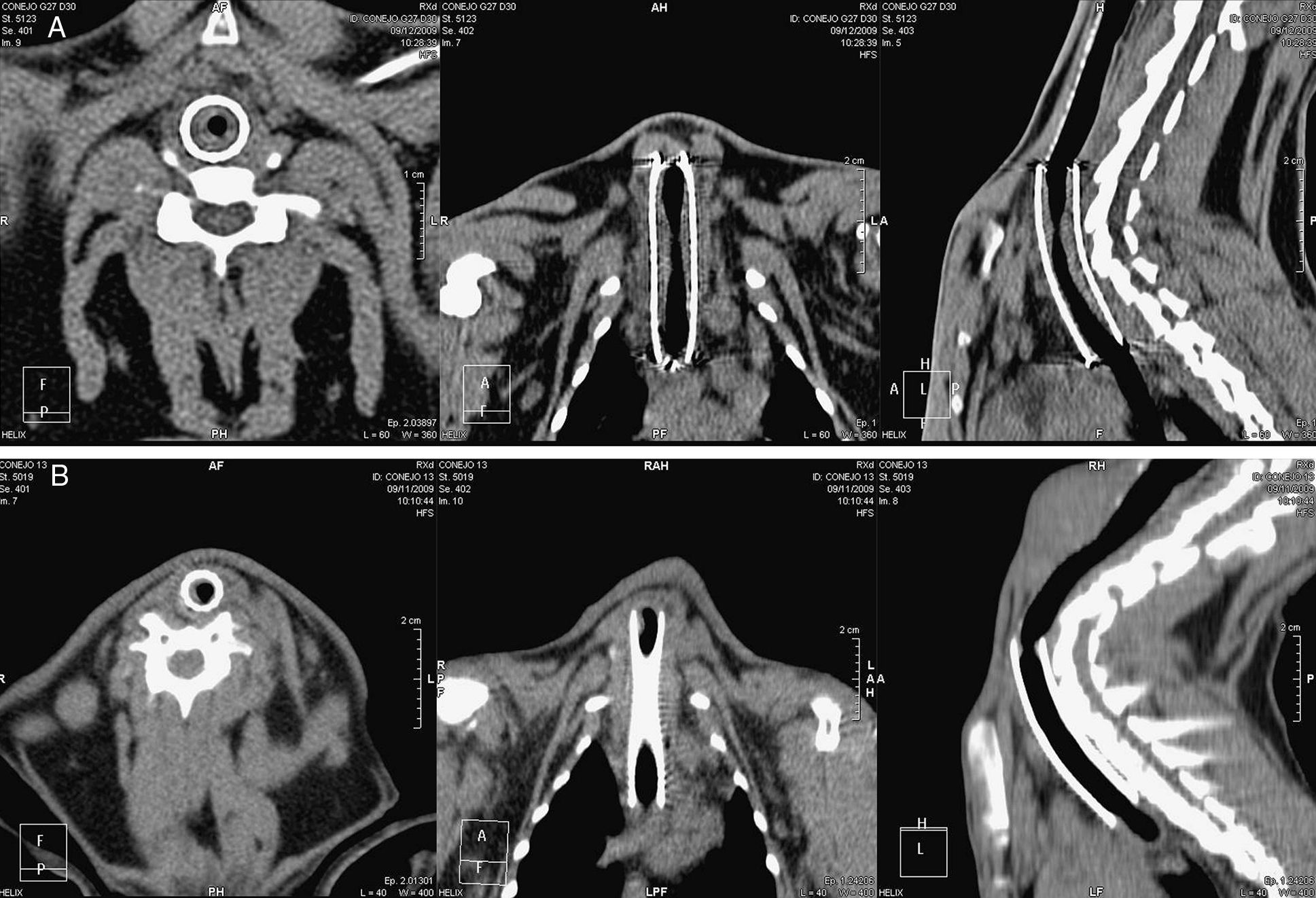
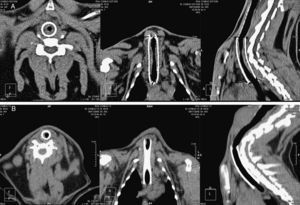
CT tracheal studies were performed under sedation 30 and 90 days after stent deployment (Phillips Brilliance CT 16-slice, The Netherlands). CT scans were performed with 1mm slice thickness, 0.5mm inter-slice gap, pitch 0.69, and three dimensional reconstructions were obtained.
Parameters assessed in the CT study were: maximum tracheal lumen stenosis in an axial view (%), stenosis length in a sagittal view (mm), and detection of image compatible with granulomas (present or absent).
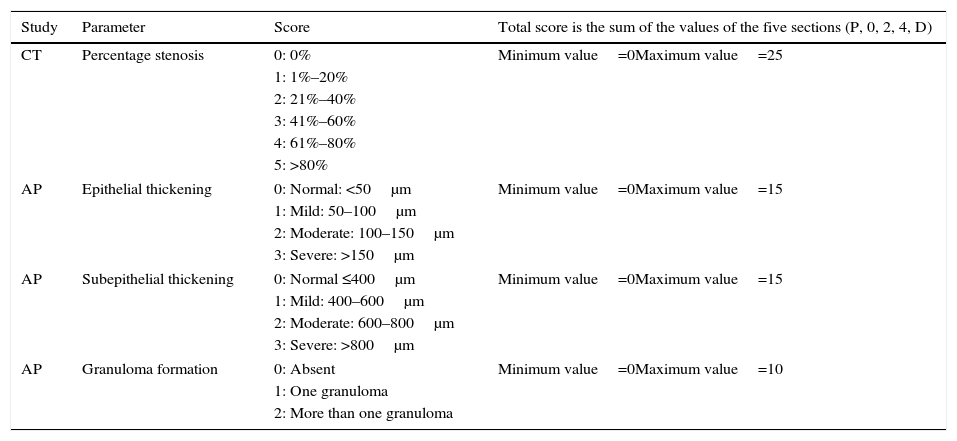
A stenosis score was also calculated, as described in Table 1, to better evaluate the entire trachea. Five sections in each trachea were defined to simplify CT interpretation: P, tissue immediately cranial to the proximal end of the stent; 0, proximal end of the stent; 2, central part of the stent; 4, distal end of the stent; and D, tissue immediately caudal to the distal end of the stent.
Scores in CT and AP Studies.
| Study | Parameter | Score | Total score is the sum of the values of the five sections (P, 0, 2, 4, D) |
|---|---|---|---|
| CT | Percentage stenosis | 0: 0% | Minimum value=0Maximum value=25 |
| 1: 1%–20% | |||
| 2: 21%–40% | |||
| 3: 41%–60% | |||
| 4: 61%–80% | |||
| 5: >80% | |||
| AP | Epithelial thickening | 0: Normal: <50μm | Minimum value=0Maximum value=15 |
| 1: Mild: 50–100μm | |||
| 2: Moderate: 100–150μm | |||
| 3: Severe: >150μm | |||
| AP | Subepithelial thickening | 0: Normal ≤400μm | Minimum value=0Maximum value=15 |
| 1: Mild: 400–600μm | |||
| 2: Moderate: 600–800μm | |||
| 3: Severe: >800μm | |||
| AP | Granuloma formation | 0: Absent | Minimum value=0Maximum value=10 |
| 1: One granuloma | |||
| 2: More than one granuloma | |||
CT: computed tomography; AP: anatomical pathology study; P, 0, 2, 4 D: tracheal sections assessed (P, tissue immediately cranial to the proximal end of the stent; 0, proximal end of the stent; 2, central part of the stent; 4, distal end of the stent; and D, tissue immediately caudal to the distal end of the stent).
CT studies were blindly reviewed by two senior radiologists who scored each case independently. In cases of disagreement, a third observer was consulted.
AP StudyAnimals were sacrificed at day 90 by intravenous sodium pentobarbital injection (Dolethal®, Vétoquinol, France), and the trachea was prepared for AP studies.
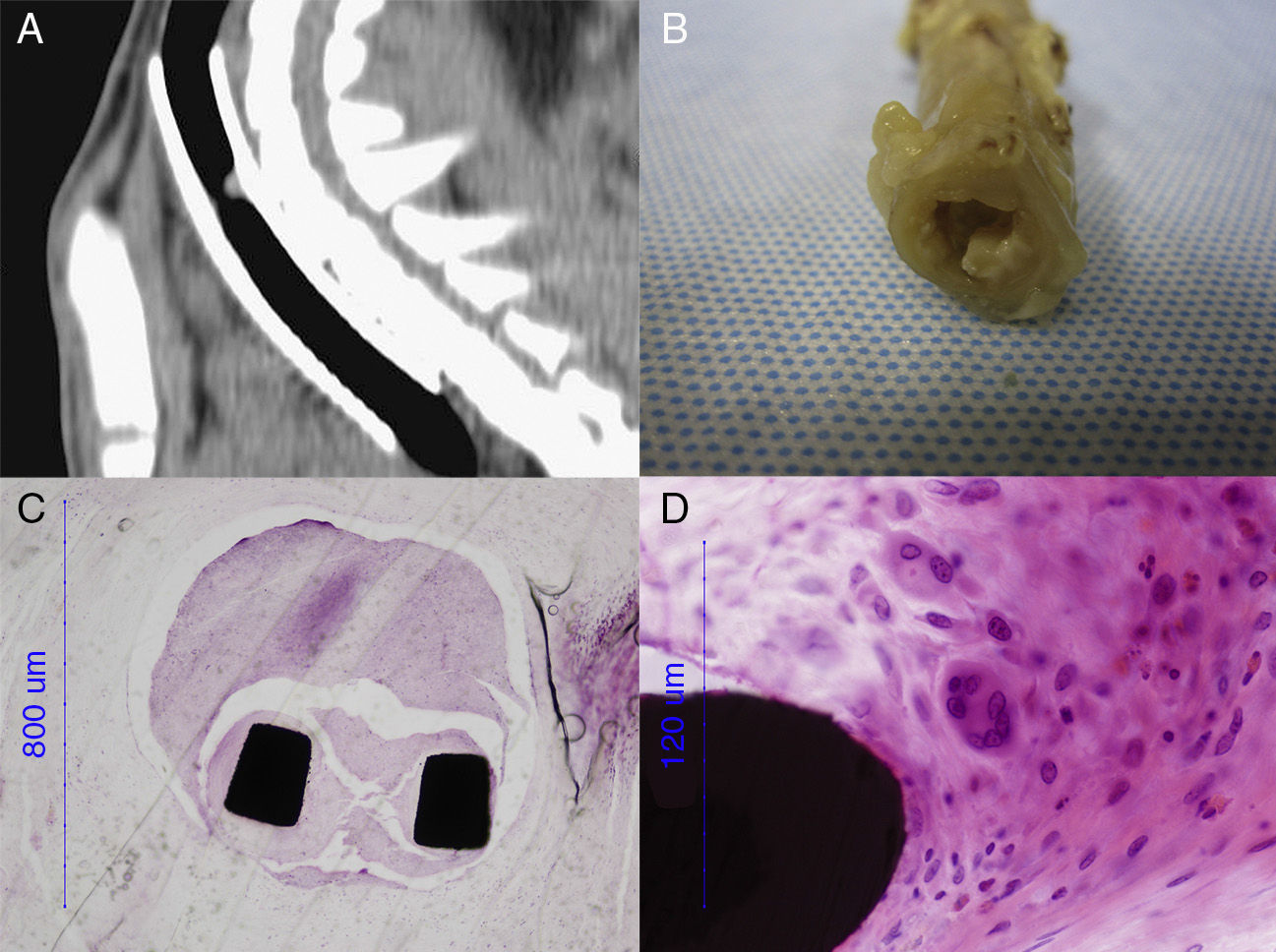
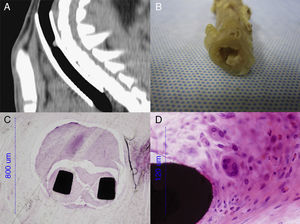
The entire trachea was fixed in 10% formaldehyde. The part containing the metal stent was embedded in methyl methacrylate, cut by a diamond band saw microtome (EXAKT 310 CS/CP, Norderstedt, Germany), and polished into 8μm-thick sections (EXAKT 400CS Norderstedt, Germany). The segments immediately cranial and caudal to the stents and the entire trachea of the control group were paraffin-embedded, and cut into 3–5μm-thick sections (Leica Reichert-Jung BIOCUT 2030 Microtome, Wetzlar, Germany). Samples were stained with hematoxylin–eosin and studied using an optical microscope (Nikon Eclipse 80i, Nikon Instruments Europe).
The same five sections (P, 0, 2, 4, D) defined for the CT study were also used for the histological study. Parameters studied in histology were: epithelial and subepithelial thickness, changes in respiratory epithelium, squamous metaplasia, granuloma formation, and inflammation and vascularization. Parameters were scored (where possible) according to Table 1.
Statistical AnalysisAll data processing and statistical analyses were performed using SPSS Statistics 17.0 for Windows. An α error of 0.05 was established. Qualitative variables were expressed as frequencies, while quantitative variables were described as mean±standard deviation. Qualitative variables were compared using the Likelihood Ratio test.
Before comparison, normality of quantitative variables was tested using the Kolmorov–Smirnov test. If the data were normally distributed, the Student's t-test for independent samples (two means) or analysis of variance, ANOVA (more than two means), were applied. For non-normal distributions, the Mann–Whitney U test or Kruskal–Wallis tests were applied. In cases of ordinal variables, the Wilcoxon test was used.
Correlations were assessed using Spearman's correlation coefficient (ρ) and the corresponding coefficients of determination (ρ2).
ResultsThe technical success of implantation was 100%. There were no immediate deaths or major complications due to this procedure.
Although most animals were sacrificed at day 90, eight animals (80%) in the ST group died before the end of the study (mean: 31.4 days post-intervention); 2 animals (20%) died in the NiTi group (mean: 39.5 days); and 4 animals (40%) in the DES group died (mean: 14.5 days). All deaths were due to stent-related causes, such as stenosis and infection.
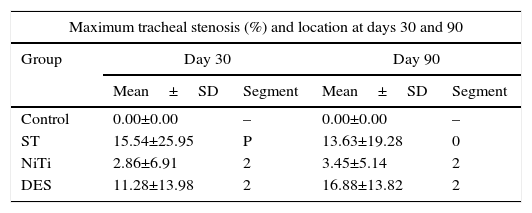
CT StudyStenosis GradingThe maximum tracheal stenosis (%) and its location at days 30 and 90 are shown in Table 2. While maximum tracheal stenosis was located at the proximal end in the steel group, the central segment was more affected in the nitinol group (Fig. 2). Stenosis scores in each group are summarized in Table 3. The ST and DES groups developed higher, but not statistically significant, scores at both days.
Stenosis Assessment in the CT Study.
| Maximum tracheal stenosis (%) and location at days 30 and 90 | ||||
|---|---|---|---|---|
| Group | Day 30 | Day 90 | ||
| Mean±SD | Segment | Mean±SD | Segment | |
| Control | 0.00±0.00 | – | 0.00±0.00 | – |
| ST | 15.54±25.95 | P | 13.63±19.28 | 0 |
| NiTi | 2.86±6.91 | 2 | 3.45±5.14 | 2 |
| DES | 11.28±13.98 | 2 | 16.88±13.82 | 2 |
| Stenosis length (mm) in CT scans at days 30 and 90 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Group | Day 30 | Day 90 | ||||||
| n | Mean±SD | Min | Max | n | Mean±SD | Min | Max | |
| ST | 6 | 6.57±6.54a | 0.0 | 16.5 | 2 | 14.55±12.52a | 5.7 | 23.4 |
| NiTi | 8 | 5.84±12.43a | 0.0 | 36.0 | 8 | 8.19±11.64a | 0.0 | 34.7 |
| DES | 8 | 20.51±14.08b | 0.0 | 39.2 | 6 | 21.62±3.94a | 16.0 | 28.2 |
| P-value* | .043 | .065 | ||||||
ST, steel stent group; NiTi, nitinol stent group; DES, nitinol drug eluting stent group. P, tissue immediately cranial to the proximal end of the stent; 0, proximal end of the stent; 2, central part of the stent.
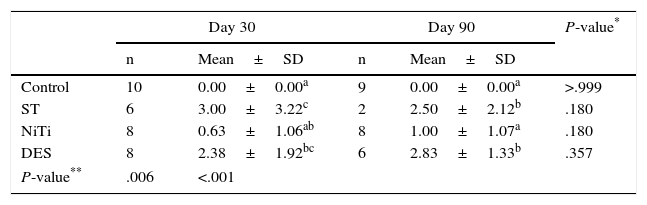
Tracheal Lumen Stenosis (Scores, Maximum=25).
| Day 30 | Day 90 | P-value* | |||
|---|---|---|---|---|---|
| n | Mean±SD | n | Mean±SD | ||
| Control | 10 | 0.00±0.00a | 9 | 0.00±0.00a | >.999 |
| ST | 6 | 3.00±3.22c | 2 | 2.50±2.12b | .180 |
| NiTi | 8 | 0.63±1.06ab | 8 | 1.00±1.07a | .180 |
| DES | 8 | 2.38±1.92bc | 6 | 2.83±1.33b | .357 |
| P-value** | .006 | <.001 | |||
Values with the same superscript indicate no statistical difference between values according to the paired Mann–Whitney U test.
At day 30 post-implantation, the DES group presented significantly longer stenosis than the other two groups. In all groups, the stenosis length increased at day 90, but stenosis length in the ST and NiTi groups increased more than in the DES group (Table 2). There were no statistical differences between the groups at day 90, or between days 30 and 90 within the same group.
Granuloma FormationAll groups appeared to have granulomas, which were located in segments 0 and/or 2 (Fig. 3). Scans from the DES group had the greatest intensity of suspected granulomas. The percentage of cases in the DES group with an apparent granuloma was statistically significant in segment 2 at day 30 (50%, P=.010), and in segment 0 at day 90 (50%, P=.018). There were no statistically significant differences in the other groups.
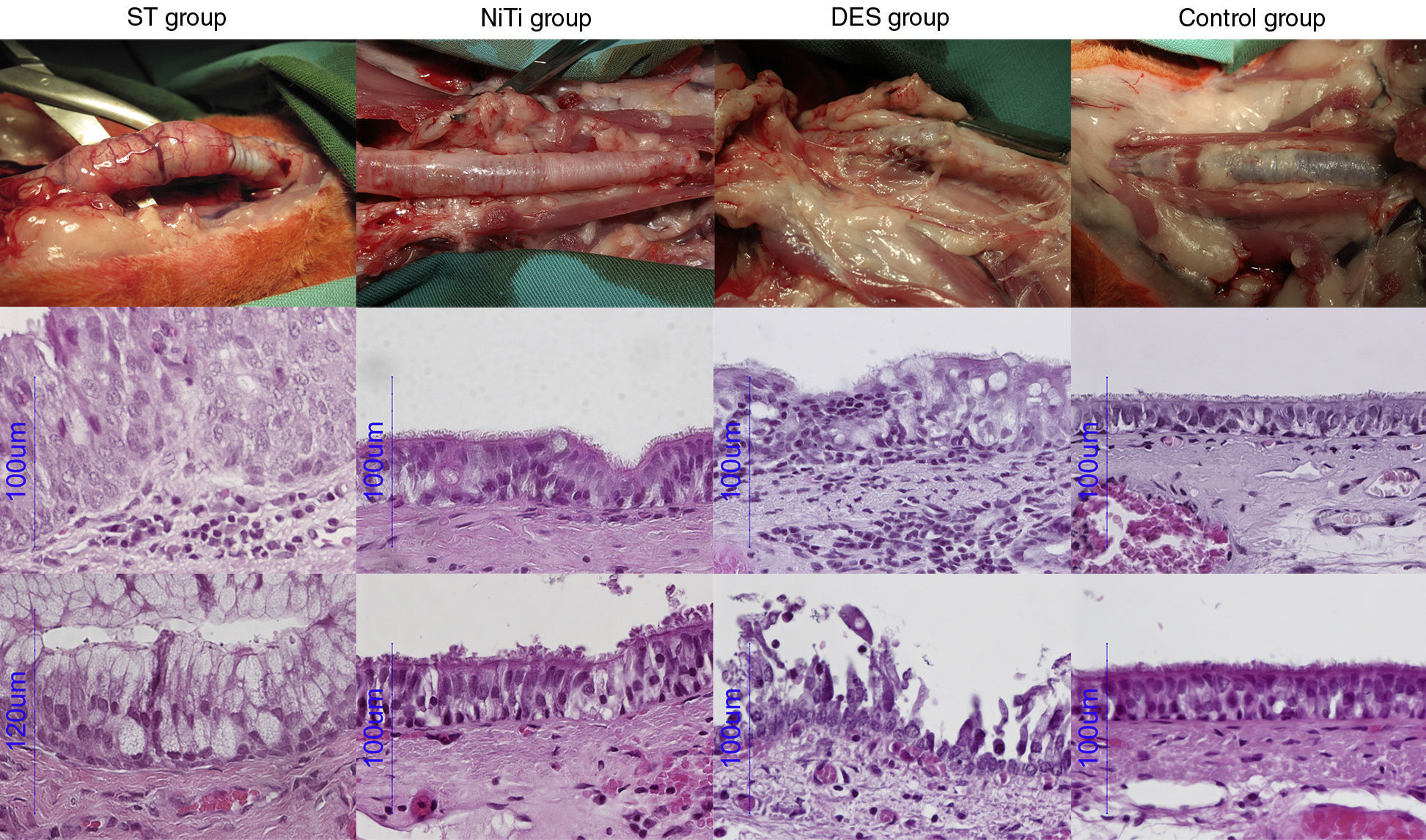
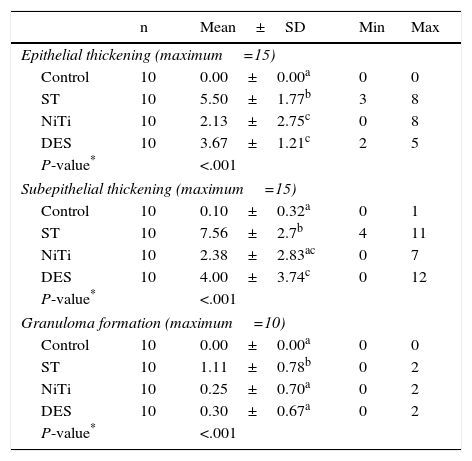
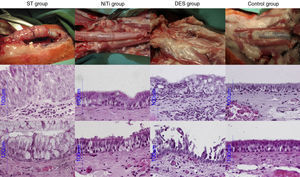
AP StudyAll AP scores are summarized in Table 4 and AP images are presented in Fig. 4.
Assessment of Histological Parameters (Scores).
| n | Mean±SD | Min | Max | |
|---|---|---|---|---|
| Epithelial thickening (maximum=15) | ||||
| Control | 10 | 0.00±0.00a | 0 | 0 |
| ST | 10 | 5.50±1.77b | 3 | 8 |
| NiTi | 10 | 2.13±2.75c | 0 | 8 |
| DES | 10 | 3.67±1.21c | 2 | 5 |
| P-value* | <.001 | |||
| Subepithelial thickening (maximum=15) | ||||
| Control | 10 | 0.10±0.32a | 0 | 1 |
| ST | 10 | 7.56±2.7b | 4 | 11 |
| NiTi | 10 | 2.38±2.83ac | 0 | 7 |
| DES | 10 | 4.00±3.74c | 0 | 12 |
| P-value* | <.001 | |||
| Granuloma formation (maximum=10) | ||||
| Control | 10 | 0.00±0.00a | 0 | 0 |
| ST | 10 | 1.11±0.78b | 0 | 2 |
| NiTi | 10 | 0.25±0.70a | 0 | 2 |
| DES | 10 | 0.30±0.67a | 0 | 2 |
| P-value* | <.001 | |||
Values with the same superscript indicate no statistical difference between values according to the paired Mann–Whitney U test
ST Group: The gross AP result of the ST group was characterized by thickening of the tracheal wall and significant formation of new vessels. There was an increase in goblet cells and a decrease in ciliated cells. The percentage of cases of squamous metaplasia was statistically higher in this group throughout the trachea (P<.001). Granuloma formation, mainly observed at the ends of the stent, was also statistically higher in this group (44.4% of cases), and in fact, this was the only group in which more than one granuloma was observed in the same section (11.1% of cases). Acute and chronic inflammation were also observed in this group, but only acute inflammation was statistically higher than the other groups (P<.001). New vessel formation was also statistically higher in the ST group (between 22.2% and 55.6% of cases; P<.001), compared to the other groups.
NiTi Group: The nitinol stent caused fewer cases of epithelial and subepithelial thickening, which was mild to moderate in appearance, but not severe. The proportion of goblet and ciliated cells was mostly maintained, and in cases of alteration, the disproportion was mild and primarily located at the ends of the stent. We observed squamous metaplasia in segment 4 of 25% of cases, although it was not statistically significant. Few granulomas were detected (12.5% and 10%, at segments 4 and D, respectively) in this group, and less inflammation and neovascularization were observed.
DES Group: DES caused destruction of the tracheal structure in 7 animals (70%), and stent struts were observed throughout the tracheal wall, with adherence to surrounding tissue. The epithelium was destroyed in a significant number of cases (40% in the proximal part, 60% in the distal part and 30% in the parts in contact with the stent). Although the epithelium was not as thick in the DES group as in the ST group, it was thicker than in the NiTi group, and showed statistically greater changes due to destruction (P<.001). Overall, squamous metaplasia was not as significant in the DES group as in the ST group, although it was statistically higher in the central segment (40% of cases, P=.020). Granulomas were detected in segment 0 in 10% of the cases, and in segments 4 and D in the same proportion as the NiTi group. Inflammation was primarily composed of lymphocytes and histiocytes, suggesting a chronic response. Neovascularization was also higher in the DES group compared to the NiTi group, but lower than in the ST group.
Correlation Between the CT and Histological StudiesA positive correlation was found between epithelial thickening (as observed in the microscopic study) and stenosis grade (as detected by CT) at day 90 in 68.9% of cases. However, there was no statistically significant correlation between the two techniques for detection of granulomas (P=.880).
ConclusionsThe treatment of choice for symptomatic lesions of the airway is surgical resection, but other treatment alternatives are sometimes required, with or without placement of silicone stents. Stenting is an alternative for tracheobronchial lesions in which surgery is not possible. The use of self-expandable or balloon-expandable metal stents has extended to the treatment of obstructive or stenotic diseases throughout the body. Many doctors are increasingly deploying expandable metal stents for the management of patients with diverse endobronchial disorders.13
The self-expandable nitinol stent has shown the best results in both benign and malignant lesions of the tracheobronchial tree.14,15 However, there have been no comparative studies or series of sufficient sample size to substantiate these results. Our study compared two different types of metal stents (steel and nitinol alloy). Due to the favorable results of DES in avoiding restenosis in other areas of the body, a third nitinol stent, coated with paclitaxel, was also included.
The rabbit trachea is known to be very reactive, so this species was chosen to assess tracheal wall responses to metal stents.16 Furthermore, percutaneous stenting in this animal model is an easy, quick and effective procedure. The five different segments that were analyzed in each sample were useful for evaluating the entire tracheal reactivity and stenosis location.
Steel stents were first used in the tracheobronchial tree with initially favorable results, but shortcomings were quickly revealed.3,17,18 De Gregorio et al. reported that three out of four steel stents used to treat benign lesions in the trachea had to be removed a year after their deployment.19 These findings were corroborated in our study, and in fact, mortality was highest in the steel stent group (80%) due to stenosis and infection. Foreign body response and mucus accumulation increased the likelihood of infection. Pasteurella multocida pneumonia was found at necropsy, proliferating due to the poor condition of the airway of the animals, caused by stent deployment. Tracheal stenosis and granulomas in our CT studies were, as expected, more significant in the ST group compared to the NiTi group.
Bare nitinol self-expanding stents perform best in both benign and malignant stenotic lesions of the tracheobronchial tree. Several authors have reported acceptable results with this type of stent.8,20–22 Our study also shows a low rate of epithelial lesions and granulomas in tracheas treated with bare nitinol stents.
The main cause of restenosis, post-stenting, is tracheal wall thickening due to granuloma formation.23,24 Our initial hypothesis was that a paclitaxel DES would improve the results since epithelial reaction would be reduced. However, 50% of DES cases developed granulomas in the central segment of the stent at day 30 (P=.010), and in segment 0 at day 90 (P=.018) in the CT study. Notable epithelial destruction was also observed in this group. These results are comparable to the in vitro study by Arellano at al., in which these DES were found to provoke prompt and significant cellular destruction in fibroblast cultures.25
Few studies of DES stents in the treatment of airway stenosis have been performed. One report presented a positive effect of a mitomycin C-eluting, bioabsorbable stent implanted in the trachea of rabbits, in comparison to four other types of stents.26 Several authors have also used paclitaxel-eluting stents in the treatment of patients with emphysema to maintain airway patency.27–30 Although the results were initially promising, the improvement was only temporary. Except for airway bypass stents for emphysema, DES are not clinically indicated for airway stenting. Future studies should perhaps focus on how to prevent granulomas and reduce stent colonization with bacteria and fungi, etc.
Our comparative study was consistent with published results for the steel and bare nitinol stents, but our findings in the DES group were surprisingly negative. DES caused severe alterations in all layers of the tracheal wall, even affecting the cartilage. This could be because the conditions of a DES differ according to location. The correct drug dosage is also important in order to avoid tissue damage.31 In another study, high paclitaxel concentration was associated with a poorer response in the bile duct.32 The bile duct and tracheal wall both have different histological structures compared to blood vessels, where the use of DES has been most effective. Furthermore, unlike in other locations, blood flow produces a continuous lavage of drug, which may explain the paclitaxel overdose in the airway in our study. Moreover, the radial forces exerted by the stents in the airway lumen against the airway wall are significant in this model. Nevertheless, the design and size of the DES and the NiTi are the same, suggesting that any differences observed are due to the drug.
The main limitation of this study is that it was conducted in laboratory animals. Tracheal anatomy and reactivity of the rabbit is similar, but not identical, to humans. Moreover, due to the small size of the airway, we used stents commercialized for vascular use, with a fixed drug concentration. Data from day 90 in the ST group is probably not representative because of the high mortality prior to the end of the study (80%).
In conclusion, our study confirms the previously reported effects of steel stents, which caused granulomas and significant stenosis. Nitinol stents were the least reactive of those assessed. DES caused significant lesions with destruction in all layers of the trachea, possibly related to drug dosage.
FundingThis study was funded by Fondo de Investigación Sanitaria del Instituto de Salud Carlos III research grant (number PI081424).
Conflict of InterestThe authors declare no conflict of interest.
The guarantor of this study is Miguel Angel de Gregorio, principal investigator of the Minimally Invasive Techniques Research Group of the University of Zaragoza.
Please cite this article as: Serrano C, Lostalé F, Rodríguez-Panadero F, de Blas I, Laborda A, de Gregorio MA. Stents traqueales metálicos autoexpandibles. Estudio comparativo de 3 tipos diferentes de stents en un modelo animal. Arch Bronconeumol. 2016;52:123–130.