Chronic rhinosinusitis with nasal polyposis (CRSwNP) is a chronic inflammatory disease that affects about 5% of the general global population.1 It constitutes a major health problem with significant comorbidities and high socioeconomic and healthcare costs.2 Some co-existing medical conditions (such as moderate or severe asthma, aspirin-exacerbated respiratory disease (AERD), and allergic rhinitis) are known to contribute to worsening symptoms3; moreover, these conditions represent the most severe and difficult-to-treat CRSwNP phenotype, and their symptoms often may often not respond to conventional treatments.4,5 Further, the cellular and molecular mechanisms underlying the clinical phenotypes of CRSwNP have not been fully understood.
Thus, even though the role of innate inflammatory cells (eosinophils versus neutrophils) and the presence of differentiated T-effector cells (Th1, Th2, and Th17) have been well established in Europe and Asia,6,7 and regulatory T cells have been proposed as a key factor in the formation of nasal polyps (NP),8 controversy and gaps remain in the knowledge regarding the modulation mechanism involved and the role associated to different subtypes of CRSwNP, as well as their clinical significance.
We report for the first time a different and critical activation state in the lymphocytes of the peripheral blood-nasal polyp-non-polypoid adjacent mucosa axis from CRSwNP patients, compared with peripheral blood-healthy mucosa from control subjects. Moreover, given that more than one comorbidity frequently appeared concomitantly in the CRSwNP patients, as cited above, we focused on the T cell population study in CRSwNP patients without any associated comorbidity and CRSwNP who also suffered from asthma, allergy, and AERD.
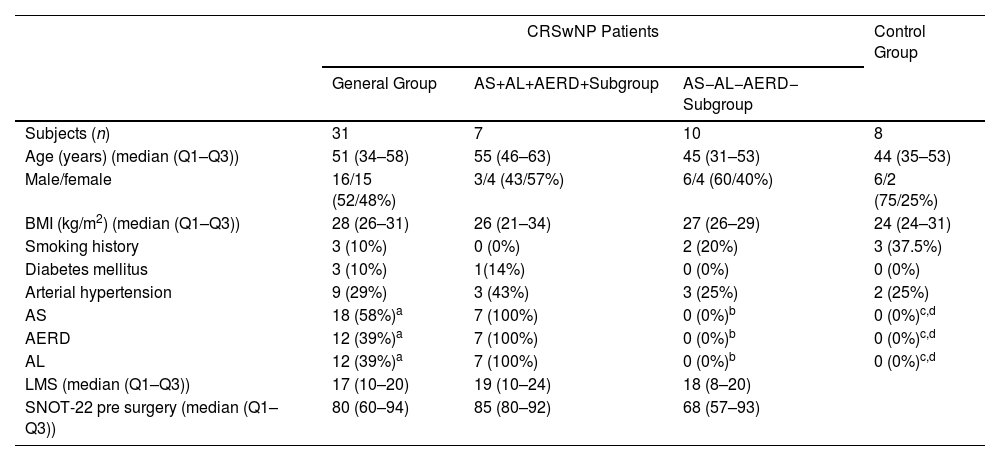
In the CRSwNP patients and controls, we analysed the immunophenotype of T cells and their level of tissue activation using flow cytometry. For this, specimens of NP and adjacent nasal mucosa were dissected from the left-middle meatus of thirty-one CRSwNP patients with severe CRSwNP (following EPOS criteria).3 All patients fulfilled the RAND-UCLA criteria for endoscopic sinus surgery.9 Moreover, specimens of healthy nasal mucosa were obtained from the uncinate tissue (left nostril) from eight controls subjects with no allergic, nasal, bronchial, or systemic inflammatory diseases during a septoplasty. Then, fresh NPs and mucosa samples were digested, and stromal vascular cells (SVCs) were separated by centrifugation.10 Furthermore, PB was collected from the CRSwNP patients and control subjects before surgery. The demographic and clinical variables of the patients and control group are shown in Table 1. All the samples were immunophenotypically characterized by flow cytometry to determinate the proportion of regulatory cellular subpopulations, which are described as CD4CD25CD127Low/− and CD8CD25CD127Low/− T cells (Figures S1 and S2). Further methods details are included in the supplementary data.
Demographics and Clinical Characteristics of the Study Subjects.
| CRSwNP Patients | Control Group | |||
|---|---|---|---|---|
| General Group | AS+AL+AERD+Subgroup | AS−AL−AERD− Subgroup | ||
| Subjects (n) | 31 | 7 | 10 | 8 |
| Age (years) (median (Q1–Q3)) | 51 (34–58) | 55 (46–63) | 45 (31–53) | 44 (35–53) |
| Male/female | 16/15 (52/48%) | 3/4 (43/57%) | 6/4 (60/40%) | 6/2 (75/25%) |
| BMI (kg/m2) (median (Q1–Q3)) | 28 (26–31) | 26 (21–34) | 27 (26–29) | 24 (24–31) |
| Smoking history | 3 (10%) | 0 (0%) | 2 (20%) | 3 (37.5%) |
| Diabetes mellitus | 3 (10%) | 1(14%) | 0 (0%) | 0 (0%) |
| Arterial hypertension | 9 (29%) | 3 (43%) | 3 (25%) | 2 (25%) |
| AS | 18 (58%)a | 7 (100%) | 0 (0%)b | 0 (0%)c,d |
| AERD | 12 (39%)a | 7 (100%) | 0 (0%)b | 0 (0%)c,d |
| AL | 12 (39%)a | 7 (100%) | 0 (0%)b | 0 (0%)c,d |
| LMS (median (Q1–Q3)) | 17 (10–20) | 19 (10–24) | 18 (8–20) | |
| SNOT-22 pre surgery (median (Q1–Q3)) | 80 (60–94) | 85 (80–92) | 68 (57–93) | |
CRSwNP: chronic rhinosinusitis with nasal polyps; Q1–Q3: first and third quartile; BMI: body mass index; AS: asthma; AL: allergy; AERD: aspirin exacerbated respiratory disease; LMS: Lund–Mackay score; SNOT-22: Sino-nasal Outcome Test-22 items.
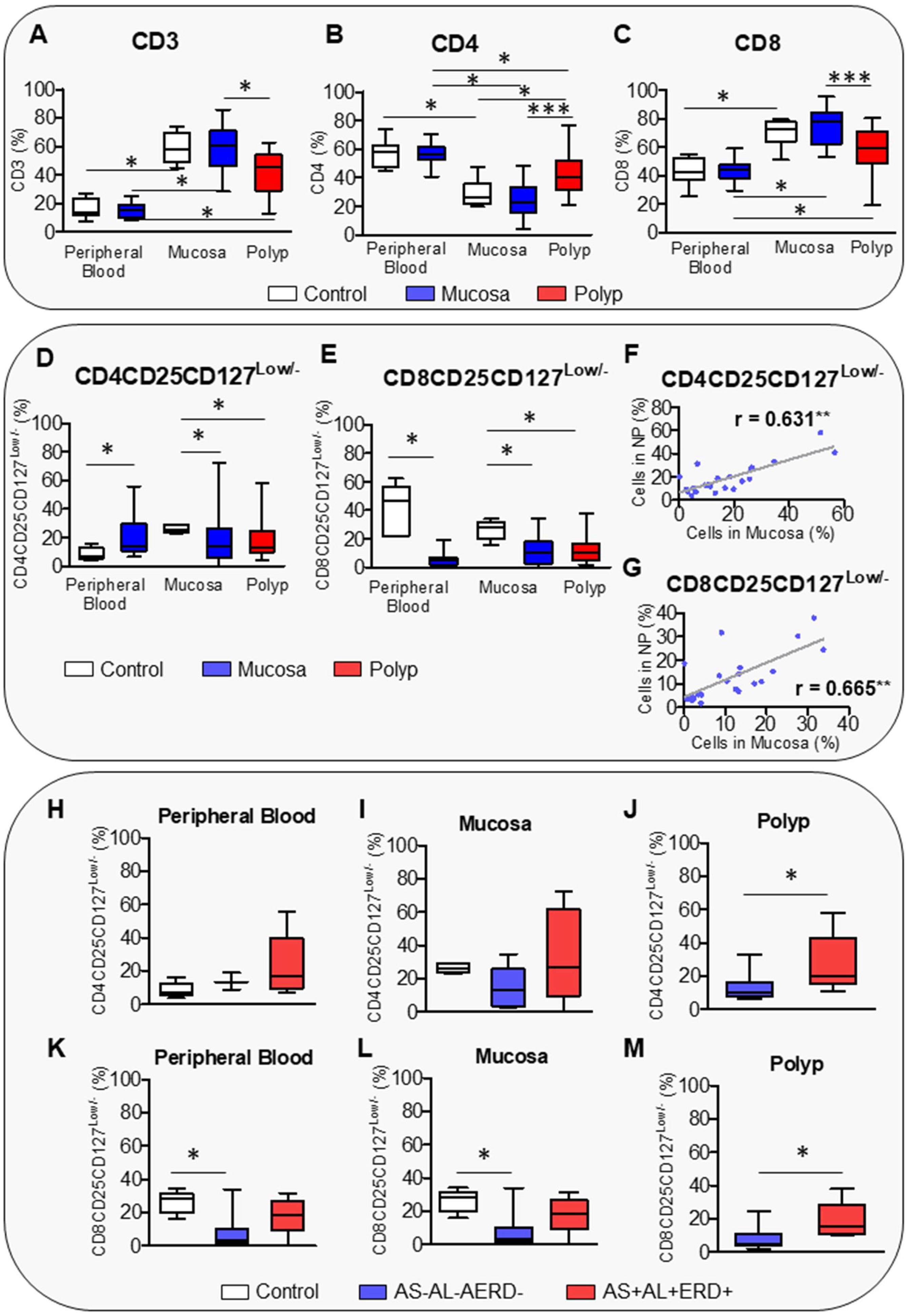
The results showed that tissue samples presented a higher percentage of CD3 and CD8 cells but a lower percentage of CD4 cells than PB (Fig. 1A–C). PB and nasal mucosa from the CRSwNP patients presented similar levels of T cells to the control subjects. Curiously, NPs showed less infiltration of CD3 and CD8 but more infiltration of CD4 cells than the control mucosa and matched non-polypoid adjacent mucosa (Fig. 1A–C). This result is in contrast with Pant H. et al, but it should be noted that these authors did not assess whether the difference in CD8 and CD4 T cells between NP and healthy mucosa persists in the adjacent mucosa of the same subject.11 Such assessment helps to clarify whether this alteration is unique to the polyp or occurs in both tissues, thus, being an exclusive characteristic of the pathology. Ho et al. reported that the percentage of CD8 cells was not significantly different between sinus mucosa and polyp tissue, showing that only activated CD8 T cells were significantly increased in polyp tissue.12 In this sense, we observed that the CRSwNP patients had a significantly higher percentage of circulating CD4CD25CD127Low/− T cells in PB than the controls. However, the infiltration of this CD4 regulatory T subpopulation in the nasal tissues was lower than in the control mucosa (Fig. 1D). Similarly, Kim et al. showed that the percentage of CD4CD25CD127Low/− T cells was lower in the NP of the CRSwNP patients,13 although other studies did not find any difference in regulatory T cells.12,14–16 Conversely, circulating and infiltrated CD8CD25CD127Low/− T cells were reduced in the CRSwNP patients (Fig. 1E), similar to that described for other pathologies with abnormal tissue growth such as malignancies.17,18 Moreover, a decrease in regulatory T cells was associated with tumour stage.17,18 The number of regulatory T cells decreases in early tumour stages and increases in more advanced stages, i.e., in breast cancer.19 Our results showed that the percentage of CD4CD25CD127Low/− and CD8CD25CD127Low/− T cells was decreased in both NPs and matched non-polypoid mucosa, with a significantly positive correlation between both tissues (Fig. 1F and G).
Box plot of CD4CD25CD127Low/− and CD8CD25CD127Low/− T cells characterization in CRSwNP. Analysis of percentage of CD3 (A), CD4 (B), CD8 (C), CD4CD25CD127Low/− (D), and CD8CD25CD127Low/− T cells (E) in peripheral blood, nasal mucosa, and polyp from control and CRSwNP patients. Spearman correlation of CD4CD25CD127Low/− (F) and CD8CD25CD127Low/− T cells (G) between nasal mucosa and polyp from CRSwNP patients. Differences of CD4CD25CD127Low/− T cells in peripheral blood (H), mucosa (I), and polyp (J) samples between CRSwNP patients with (AS+AL+AERD+) or without (AS−AL−AERD−) associated comorbidities and control subjects. Differences of CD8CD25CD127Low/− T cells in peripheral blood (K), mucosa (L), and polyp (M) samples between CRSwNP patients with (AS+AL+AERD+) or without (AS−AL−AERD−) associated comorbidities and control subjects. *p≤0.05; **p<0.01; ***p<0.001.
Our results are in line with that of previous studies that reported an association of high levels of regulatory T cells not only with advanced stages in different tumours, but also with future relapse,20 thus, predicting worse clinical outcomes. CD8 regulatory T cells were suggested to play a role in the mechanism of immune impairment in patients with ovarian cancer.21 In this sense, we found that a decrease in this T cell subpopulation is related with a worse quality of life in CRSwNP patients (Spearman's rho correlation coefficient between CD8CD25CD127Low/− T cells and Sino-nasal Outcome Test-22 (SNOT-22) items −0.649; p-value 0.016).
Given that more diseases can often coexist in CRSwNP patients, the inflammatory response patterns can be modified. To address this point, we analysed the CD4CD25CD127Low/− and CD8CD25CD127Low/− regulatory T cells in CRSwNP patients suffering from asthma, allergy, and AERD (AS+AL+AERD+ group), as well as CRSwNP patients without any of these associated comorbidities (AS−AL−AERD− group). The demographic and clinical variables of both groups are shown in Table 1. CRSwNP patients without concomitant morbidities showed the lowest level of circulating and infiltrated CD8CD25CD127Low/− T cells and a tendency of the lowest CD4 CD25CD127Low/− infiltration in mucosa (Fig. 1H and M). These patients also presented different response pattern in NPs, with a reduction in the infiltration of both regulatory T cells’ subpopulations (Fig. 1J and M). Finally, these results suggest that an imbalance of C4CD25CD127Low/− and CD8CD25CD127Low/− T cells plays an important role in NP formation. Furthermore, the CD8CD25CD127Low/− T cells – the most important biomarker, much like the regulatory T cells that have a well-known involvement in cancer – could be considered as a biomarker of severity and evolution for this pathology. Moreover, clinical and therapeutical implications provide advantages such as easy detection and measurement in PB, using flow cytometry analysis, a useful tool present in most tertiary hospitals. This opens a new opportunity for translational research that allows finding a better phenotypic characterization of nasal polyps prone to recurrence, oriented to the accurate applications of new treatments in these patients. Our study has some limitations, such as, all the patients were candidates for endoscopic sinus surgery; despite this, they presented a certain degree of variability in disease severity (LMS, SNOT-22). In future studies, it would be interesting to increase the number of patients and categorize them according to endoscopic, clinical, and radiologic features. This type of cell characterization would be complementary to the different endotypes proposed in the literature to date.3,22 New analyses correlating the presence of different biomarkers will be necessary in the future.
Ethical ApprovalThe study was conducted according to the guidelines of the Declaration of Helsinki, was approved Research Ethics Committee of Hospitales Universitarios Virgen Macarena-Virgen del Rocio (projects PI-0212-2017 and PIGE-0367-2019) and written informed consent has been approved by obtained from the patients and controls to publish this paper.
FundingThis work was supported by Grant PI-0212-2017, PIGE-0367-2019 and RH-0048-2021 of the “Consejería de Salud y Familias, Junta de Andalucía” Spain.
Conflicts of InterestThe authors declare no conflict of interest.
We want to acknowledge the patients and the Biobank Nodo Hospital Virgen Macarena (Biobanco del Sistema Sanitario Publico de Andalucía) integrated in the Spanish National biobanks Network (PT20/00069) supported by ISCIII and FEDER funds, and Anatomical Pathology Unit of the Hospital Universitario Virgen Macarena of Seville, especially to Maria Jose Rios Moreno for her collaboration in this work.