The general aim of this study is to create a cohort of asthma patients with varying grades of severity in order to gain greater insight into the mechanisms underlying the genesis and course of this disease.
The specific objectives focus on various studies, including imaging, lung function, inflammation, and bronchial hyperresponsiveness, to determine the relevant events that characterize the asthma population, the long-term parameters that can determine changes in the severity of patients, and the treatments that influence disease progression. The study will also seek to identify the causes of exacerbations and how this affects the course of the disease.
Patients will be contacted via the outpatient clinics of the 8 participating institutions under the auspices of the Spanish Respiratory Diseases Networking System (CIBER). In the inclusion visit, a standardized clinical history will be obtained, a clinical examination, including blood pressure, body mass index, complete respiratory function tests, and FENO will be performed, and the Asthma Control Test (ACT), Morisky-Green test, Asthma Quality of Life Questionnaire (Mini AQLQ), the Sino-Nasal Outcome Test 22 (SNOT-22), and the Hospital Anxiety and Depression scale (HADS) will be administered. A specific electronic database has been designed for data collection. Exhaled breath condensate, urine and blood samples will also be collected. Non-specific bronchial hyperresponsiveness testing with methacholine will be performed and an induced sputum sample will be collected at the beginning of the study and every 24 months. A skin prick test for airborne allergens and a chest CT will be performed at the beginning of the study and repeated every 5 years.
El objetivo general del estudio es la creación de una cohorte de pacientes con asma con distintos grados de gravedad, que permita incrementar los conocimientos sobre los mecanismos subyacentes a la génesis y evolución de esta patología.
Los objetivos específicos se centran en llevar a cabo diferentes estudios en términos de imagen, de función pulmonar, inflamación e hiperrespuesta bronquial, para determinar los eventos relevantes que dan forma a esta población asmática, los parámetros a largo plazo que pueden determinar los cambios en la gravedad de los pacientes y que tratamientos pueden influir en la progresión de la enfermedad. El estudio también tratará de identificar las causas de las exacerbaciones y cómo esto afecta a la evolución de la enfermedad.
Los pacientes serán contactados a través de las consultas externas de las 8 instituciones participantes en el marco del CIBER de Enfermedades Respiratorias. En la visita de inclusión, se realizará una historia clínica estandarizada, un examen clínico exhaustivo, incluyendo la presión arterial, el índice de masa corporal, las pruebas funcionales respiratorias completas y la medición de la FENO, y se administrarán los cuestionarios Test de control del asma (ACT), Morisky Green, Cuestionario de calidad de vida en pacientes con asma (Mini AQLQ), el Cuestionario sino-nasal Outcome Test 22 (SNOT-22) y la escala de ansiedad y depresión (HAD). Para la recogida de los datos se ha diseñado una base de datos electrónica específica. Se recogerán también muestras de aire exhalado condensado, orina y sangre. Al inicio del estudio y cada 24 meses, se realizará una prueba de hiperrespuesta bronquial inespecífica con metacolina y se recogerá una muestra de esputo inducido. Al inicio del estudio se realizarán prick test a neumoalérgenos y una tomografía computarizada torácica que se repetirá a los 5 años.
Asthma is a global health problem. Prevalence of this disease has risen in recent decades, and it now thought to affect up to 358 million individuals worldwide. The problem is particularly significant in industrialized countries, which have witnessed a great increase in asthma rates in the last 50 years.1,2 Asthma currently affects between 1% and 16% of the world population,1 while prevalence in Spain ranges from 1.5% to 16.7% of the adult population and around 10% of children.3 Asthma patients have a poorer quality of life, more work and school absenteeism, and a greater comorbidity burden. As this a chronic disease, socio-economic costs are high.4
Bronchial asthma is defined as an inflammatory disease of the airways that causes bronchial hyperresponsiveness or airflow obstruction, and which is manifested by symptoms such as cough, wheezing or dyspnea.1,3 Asthma has traditionally been considered a disease associated with atopy or allergy with onset in childhood that can persist or resolve in adulthood.5 However, it is now thought of as a heterogeneous and multifactorial disease with several phenotypes, each with its own natural history and distinct response to treatment.6,7 In addition to allergic or extrinsic asthma and non-allergic or intrinsic asthma, other phenotypes have been defined in the last 20 years on the basis of clinical or physiological characteristics (severity, age at onset, grade of obstruction, resistance to treatment), triggering factors (exercise, allergens, occupation, aspirin-induced asthma), or type of inflammation (eosinophilic, neutrophilic or paucicellular).8 These definitions focus on partial features of the disease, and while they may be helpful, they do not fully explain the complexities of asthma. Indeed, issues such as the causes of increasing asthma rates, genetic susceptibility, or the interaction between environmental factors and the immune system, both in the genesis of asthma or as a trigger of exacerbations, have yet to be resolved.
Two strategies have been developed in the past 10 years to improve understanding of this condition. The first is to classify asthma according to phenotypes, using cluster analysis.9–11 Cluster analysis is based on multivariate mathematical algorithms that quantify similarities between individuals of the same population, in order to define the mechanisms of the disease and, thus, optimize treatment. The second strategy consists of the creation of different patient cohorts, in order to study the natural history of the disease. To date, 3 European cohorts,12–14 and 1 American cohort15 have been developed. The purpose of these cohorts is to optimize treatment according to the particular features of each patient. However, cohorts published to date focus on the study of severe or refractory asthma. To the best of our knowledge, no cohort studies have yet focused on the analysis of the overall asthma population. Moreover, none of these cohorts includes both lung function and inflammatory studies, nor has progressive bronchial imaging been collected.
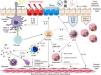
This aim of this study, the MEGA project (Mechanism underlying the genesis and evolution of asthma), is to create a cohort that would give access to clinical, physiological, molecular, and genetic data in patients with varying degrees of asthma severity. The data collected will help establish the different physiopathological pathways that cause or impact on the heterogeneous expression of this disease, and will show what percentage of patients might progress to bronchiectasis or fixed bronchial obstruction, and identify factors that might promote or influence this progress. This is a multicenter study that will be performed in 8 hospitals, under the auspices of the Spanish Respiratory Disease Biomedical Research Network (CIBERES). Specific tests, including imaging, lung function, inflammation, and bronchial hyperresponsiveness, will be performed periodically to determine the relevant events that characterize the asthma population, the long-term parameters that can determine changes in severity, and the treatments that influence disease progression. The study, which is the first of its kind, will also seek to identify the causes of exacerbations and how they affect the course of the disease. In short, the aim is to improve our understanding of the natural history of the disease, without focusing exclusively on severe asthma (Fig. 1).
This article describes the cohort selection criteria, baseline characteristics and progress-related parameters that will be collected, as well as the methods used to obtain, process, and store the various biological samples in the CIBERES pulmonary biobank.
MethodsDesign, Study Population and Clinical VariablesThis is a prospective multicenter cohort study with an estimated representative sample of 525 adult patients with asthma and 100 healthy controls. Subjects will be recruited from 8 university hospitals in Spain.
Patient recruitment will be consecutive and non-selected, with an expected loss-to-follow-up rate of 12.5%. The first 75 patients with asthma seen during the study period in each hospital, aged 18–75 years, with intermittent, mild, moderate, or severe asthma, according to the GINA classification,1 who have been diagnosed (also based on the GINA criteria) at least 1 year before inclusion, will be recruited. Patients will be excluded only if they have other acute or chronic active lung disorders, or significant psychiatric disorders. All patients will give informed consent in writing. Individuals will be contacted via the outpatient clinic of the participating institutions, and participants will be seen at the start of the study, every 6 months during the first year, and then every year. A control group of 100 healthy subjects, non-smokers, matched in age and gender with controls, will be recruited. The control group will undergo the same tests and evaluations as the asthma patients.
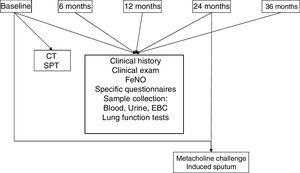
Common data collection methods will be used in all participating centers. At each visit, a standardized clinical history will be completed for each patient, and validated versions of the following questionnaires will be administered: Asthma Control Test (ACT),16,17 Morisky Green,18,19 Asthma Quality Of Life Questionnaire (20.21 Mini AQLQ),20,21 Sino-Nasal Outcome Test 22 (22.23 SNOT-22)22,23 and Hospital Anxiety and Depression (HAD).24,25 A detailed clinical examination, including blood pressure, body mass index, full respiratory function tests, and the measurement of fractional exhaled nitric oxide (FeNO), will be performed. Exhaled breath condensate, urine and blood samples will also be collected. To ensure homogeneous sample collection, common protocols have been designed for use in all participating centers. Non-specific bronchial hyperresponsiveness testing with methacholine will be performed at the beginning of the study and every 24 months, and an induced sputum sample will be collected. Chest computed tomography (CT) and skin prick tests (SPT) for airborne allergens will be performed at the beginning of the study and every 5 years thereafter (Fig. 2).
Variables and samples collected in the different study phases. The study will run for at least 10 years. Chest CT will be performed at baseline and every 5 years. Methacholine challenge and induced sputum will be performed at baseline and every 2 years. CT: computed tomography; EBC: exhaled breath concentrate; SPT: skin prick tests.
A specific electronic database and case report form (CRF) has been designed to collect study data. This database will be used to collect, manage, and export previously anonymized data from the study patients. Database variables have been agreed by a multidisciplinary team of project investigators, and the CRF was designed by a specialized company (Biostatistics and Data Management Core Facility, IDIBAPS, Institut d’Investigacions Biomèdiques August Pi i Sunyer) (Annex 1, online supplement). The company responsible for CRF design and the project investigators will perform periodic reviews to ensure the quality of the data included in the database. These reviews will take place in the first month, and every six months thereafter.
AtopyAtopy will be defined as the presence of at least 1 SPT or specific serum IgE positive for an airborne allergen.26 SPTs will be performed for the most common airborne allergens in each study region, and must include: house dust mites (Dermatophagoides pteronyssinus, Dermatophagoides farinae, Lepidoglyphus destructor), pollens (Cupressus arizónica, Platanus acerifolia, Olea europea), grasses (Artemisia vulgaris, Parietaria judaica, Salsola kali), dander (cat, dog), molds (Alternaria alternata, Aspergillus fumigatus, Caldosporium herbarum, Penicillium notatum) and cockroach (Blatella orientalis). SPTs will be considered positive in the case of wheal of at least 3mm in diameter compared to the negative control (saline); histamine 10mg/ml will be used as a positive control.26
Serum specific IgE to common airborne allergens will be determined using a commercial kit (ImmunoCAP, Thermo Fisher Scientific, Uppsala, Sweden), following the manufacturer's instructions. A result of at least 0.35KU/l will be considered positive.
Lung Function TestsForced spirometry will be performed and analyzed according to the SEPAR guidelines.27 The reference values proposed by Roca et al.28 for the Mediterranean population will be used. Spirometry will be performed before the bronchodilator test, with the administration of 2 puffs of salbutamol through a spacer (0.1mg β2-adrenergic agonist per inhalation), and 15min after. The test will be considered positive when an increase in FEV1 or forced vital capacity (FVC) greater than 200ml is recorded, representing more than 12% of the baseline value.29 Static lung volumes will be determined by plethysmography and diffusing capacity for carbon monoxide (DLCO) will be tested using the single breath method. These studies will be conducted with MasterLab analyzer (MasterLab, Jaegger, Germany) in accordance with the proposed joint guidelines of the European Respiratory Society (ERS) and the American Thoracic Society (ATS).30,31
Bronchial hyperresponsiveness will be determined using a non-specific methacholine bronchial challenge test according to the ERS/ATS guidelines.32 The test will be considered positive if FEV1 decreases more than 20% from baseline. If this decrease is not observed after the administration of 5 puffs at a concentration of 16mg/ml, the test will be considered negative.33 The test result will be expressed as PC20 (concentration [mg/ml] of methacholine that provoked a 20% decrease in FEV1).
Determination of Fractional Exhaled Nitric OxideFeNO will be measured using a chemiluminescence analyzer (NiOx; Aerocrine; Solna, Sweden), according to the guidelines recently proposed by the ATS.34
Exhaled Breath CondensateExhaled breath condensate (EBC) will be collected using an EcoScreen device (Erich Jaegger GmbH, Würzburg, Germany), following ERS/ATS guidelines.35 Briefly, patients will be advised not to eat or drink anything for 1h before the test. They must then breathe tidally while connected to the device until a total of 150L of exhaled air has been collected. When the sample has been collected, pH will then be measured after bubbling helium through the EBC for 10min. Aliquots of 0.5ml will be prepared and stored at −80°C for future biomarker studies.
Induced SputumSputum will be induced by inhaling increasing concentrations of hypertonic saline (3%, 4%, and 5%) for 7min. It will be processed and examined as previously described by Pizzichini et al.36 The quantitative cell count of all cells and different cell types will be determined in all sputum samples. Supernatant will be frozen at −80°C.
Blood and Urine SamplesAll individuals will provide a sample of whole blood for DNA extraction (10ml), plasma (10ml), and urine. These samples will be retained for future genetic and molecular studies.
Computed TomographyAll individuals included in the study will undergo a chest CT at the start of the study and then every 5 years. This study will be performed to determine the presence of bronchiolectasis (bronchus/artery ratio >1), bronchiectasis (bronchus/artery ratio >1.5), and bronchi with thickened walls, and the bronchial wall of the apical segment of the right upper lobe near its origin (mm) will be measured.37
Storage of SamplesSerum, DNA, EBC, and sputum supernatant will be stored at −80°C in each of the recruiting centers. One of the objectives of creating this cohort is to develop a specimen biobank. These samples will be included in the already existing CIBERES biobank, where they will be stored for future use in biomarker studies.
Ethical and Legal ConsiderationsThis study has been designed according to the principles of the Declaration of Helsinki (18th World Medical Assembly, 1964) and Hong Kong (1989). The purpose of the study will be explained to each patient before their consent to participate is requested. They will receive a letter explaining the utility and clinical interest of the study, and the potential risks of participating. The research project has been approved by the Clinical Research Ethics Committee of all hospitals participating in the study in accordance with Personal Data Protection Act 15/1999, Biomedical Research Act 14/2007, and Biomedical Research Royal Decree 1716/2011.
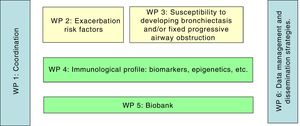
DiscussionThe main aim of creating this cohort is to contribute to the understanding of the heterogeneity of asthma. Accordingly, we will perform immunological studies to clarify the mechanisms involved in the development of differing clinical manifestations among patients, epigenetic studies to identify the factors leading to the appearance of asthma, studies to identify risk factors for exacerbations, and studies to identify patients who are susceptible to developing bronchiectasis and irreversible progressive airway obstruction.
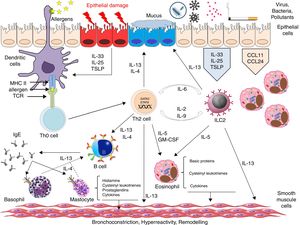
A large proportion of current research in asthma is aimed at identifying the immunological pathways that determine disease, including disease phenotypes and endotypes and certain biomarkers that may help us design precision medicine.38 It is generally agreed that 3 possible immune responses can be distinguished in the development of asthma, namely type 2 immune response, non-type 2 immune response, and mixed type Th2/Th17 immune response,39 but the intimate mechanisms that generate these responses are still largely unknown. Type 2 response is the best known, and is generally observed in asthma patients with eosinophilic inflammation.40 The distinctive feature of this response is a Th2 pathway which leads to the final production of specific IgE, the basic effector cells of which appear to be Th2 lymphocytes and plasma cells. The main cytokines involved are IL-4, IL-5 and IL-13.41 The type 2 response also occurs in non-atopic patients, apparently driven by innate T lymphocyte (ILC2), with IL-5 as the main effector cytokine.42 Before differentiation, both pathways appear to have common bronchial epithelium-derived cytokines, such as IL-33, IL-31, IL-25 and thymic stromal lymphopoietin, and these molecules are currently under investigation as possible therapeutic targets43–45 (Fig. 3). It is unclear why this second pathway, with its low Th2 profile but marked eosinophilic inflammation, is associated with more severe asthma and a poorer response to corticosteroids.
Non-type 2 immune response, also known as non-eosinophilic asthma,10,46 appears to include patients with Th17-dependent neutrophilic inflammation,47,48 patients with neutrophilic inflammation dependent on dysregulation of the innate immune response associated with IL-1b or CXCR2,49 and patients with neurogenic inflammation basically associated with the RTPA1 receptors.50,51 While these response types are the focus of several studies, the putative role of tumor necrosis factor-alpha, IL-6, IL-8, IL-37, IL-22 and IL-9 is under discussion.39
Although patients with a mixed Th17/type 2 response have been described, the relationship between the 2 response pathways is highly complex and little understood. IL-17 produced by T cells or by the Th17 cells themselves in response to possible epithelial damage was seen to increase the production of IL-4 and IL-13 by ILC2 and Th2 cells in an atopic dermatitis animal model.52 At the same time, IL-4 and IL-13 seem to be capable of amplifying the Th17 response by regulating CD209a expression in dendritic cells in murine schistosomiasis models.53 However, in murine asthma models, the neutralization of IL-4 or IL-13 results in an increase in Th17 cells and neutrophilic inflammation, while the neutralization of IL-13 and IL-17 prevents both eosinophilic and neutrophilic inflammation and the appearance of bronchial hyperresponsiveness.54 A better understanding of these pathways and their possible interactions obtained from the possible results of this cohort, may translate to a better selection of therapeutic targets and the implementation of combined treatments, such as double Th2/Th17 blockade.
Asthma is clearly a complex disease in which both genetic and environmental factors play a decisive role. However, neither genetic55 nor environmental56 factors alone can explain the variability observed in these patients. Epigenetic studies may help determine genetic-environmental interactions and may shed light on the hereditary component of asthma.57,58 Epigenetic mechanisms are known to regulate the expression of cytokines that play an important role in the differentiation of T lymphocytes and certain transcription factors,59,60 which in turn may be influenced by different environmental exposures.61 Indeed, exposure to diesel particulate matter has been associated with DNA methylation in peripheral blood and in the epithelial cells of the airways, and with altered T reg cell function when associated with exposure to polycyclic aromatic hydrocarbons.62,63 Although exposure to certain allergens has also been associated with methylation changes in the DNA of respiratory epithelial cells,62 very few studies have evaluated the influence of multiple exposures on epigenetic changes.
The interaction between changes in the microbiome and epigenetic changes is another interesting area. Epidemiological studies have described a lower incidence of asthma in individuals with less exposure to antibiotics in early life,64 and in babies delivered vaginally rather than by Cesarean section.65 In fact, there is a strong relationship between exposure to microorganisms and susceptibility to asthma in early life. Several authors believe that these early exposures may affect the composition of the microbiome of the individual and this, in turn, may generate epigenetic changes that will lead to a greater susceptibility to developing asthma.58 Although we have no plans to develop microbiome studies in this cohort, we will take into account these multiple factors that may determine epigenetic changes and, as such, may be responsible for the heterogeneity of asthma.
Little is understood about the factors that determine exacerbations in asthmatic patients. Various factors have been implicated, including respiratory infections, especially of viral origin,66–68 exposure to allergens,69 smoking,70 lack of adherence to treatment,71 rhinosinusitis,72,73 obesity, intolerance of non-steroidal anti-inflammatory drugs,74 and psychosocial factors.75 However, allergic sensitization is known to vary widely, depending on environmental and climatic conditions.76 Moreover, while viral infection seems to be a major trigger for exacerbations,66–68 the interaction between these infections and allergic sensitization associated with a specific bronchial inflammatory response has not been studied in depth, nor are studies available that associate certain factors of exacerbation with different asthma phenotypes.
While a relationship between the degree of control of asthma and the risk of exacerbations has been established,77 evidence that they are not always associated78 is supported by the fact that inflammation and bronchial hyperresponsiveness persist in patients with controlled asthma.79 It is difficult to achieve disease control in patients with severe asthma, and treatment is essentially aimed at avoiding exacerbations.3 Several markers have been proposed in an attempt to establish the risk of exacerbation, such as FEV1 or the number of exacerbations in the previous year.80 A clinical index for predicting exacerbations has recently been developed81; however, it was created from retrospective analyses and has not been fully validated, nor does it include measurements of inflammatory factors, such as FeNO or eosinophils in induced sputum, that are essential data in this disease.
Finally, some asthma patients are known to develop bronchiectasis,82 while others develop fixed airway obstruction practically indistinguishable from that experienced by patients with chronic obstructive pulmonary disease.83 Both factors predict a worse prognosis and a greater use of healthcare resources.84 These events have conventionally been associated with patients with more severe asthma or poorer disease control and a greater number of exacerbations85,86; however, the factors that determine which patients with similar clinical characteristics will go on to develop these complications remain unclear. Nor do we know if this poor prognosis can occur in less severe forms of the disease.
In conclusion, this large Spanish, prospective, multidisciplinary cohort has been created to clarify the molecular mechanisms that influence the different clinical presentations of the disease and the factors associated with certain mechanisms that may affect exacerbations or the appearance of bronchial changes in asthma patients, in the form of bronchiectasis or fixed progressive airway obstruction. Another aim is to identify potentially preventable epigenetic changes and to try to discover biomarkers to help determine the most effective treatment for each patient.
FundingThis study is funded by FIS PI15/0190 (Instituto de Salud Carlos III), the European Regional Development Fund (ERDF) and Sanofi. MJC receives funding from the Miguel Servet research program of the Instituto de Salud Carlos III (CP12/03101).
AuthorshipAll authors of the manuscript have significantly contributed to the research, preparation, review, and final version of the manuscript.
Conflict of InterestThe authors state that they have no conflict of interests.
Please cite this article as: Muñoz X, Álvarez-Puebla MJ, Arismendi E, Arochena L, del Pilar Ausín M, Barranco P, et al. Estudio de los mecanismos implicados en la génesis y evolución del asma (proyecto MEGA): creación y seguimiento a largo plazo de una cohorte de pacientes asmáticos. Arch Bronconeumol. 2018;54:378–385.