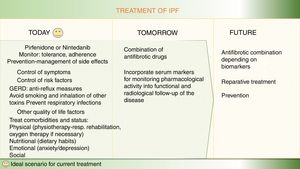
The therapeutic approach in idiopathic pulmonary fibrosis has changed substantially over the past 5 years. National and international guidelines for the pharmacological treatment of IPF recommend 2 antifibrotic drugs, nintedanib and pirfenidone. The use of both these drugs is supported by high-level evidence, with benefits including not only slower disease progression but also a reduction in the annual risk of death. Currently, the therapeutic management of these patients prioritizes both the use of drugs that act on the pathogenic mechanisms of the disease, and the positive effect of improving quality of life with integrated multidisciplinary support, including nutrition, physical activity, education, emotional support, and palliation of symptoms. The overall aim is to ensure that the patient remains as well as possible for as long as possible after diagnosis. However, the goal of the new antifibrotic combinations that are currently under evaluation in clinical trials is to use the potential antifibrotic synergy to enhance the therapeutic benefit or completely halt disease progression, by acting simultaneously on different pathogenic pathways. Another line of investigation involves markers that might be useful for identifying patients who may benefit more from certain antifibrotics than from others, which would make it possible to optimize resources and take the first steps toward precision medicine in pulmonary fibrosis. Below, we review the main potential areas for improvement in the pharmacological treatment of idiopathic pulmonary fibrosis in the short, medium, and long term.
La aproximación terapéutica en fibrosis pulmonar idiopática (FPI) ha cambiado sustancialmente en los últimos 5 años. Las guías nacionales e internacionales para el tratamiento farmacológico recomiendan el uso de dos fármacos anti-fibróticos, nintedanib y pirfenidona, con un nivel elevado de evidencia científica a favor del beneficio de ambos fármacos, no sólo por enlentecer la progresión de la enfermedad sino también por reducir el riesgo de muerte anual. Actualmente, dentro del manejo terapéutico de estos pacientes, se pone en valor tanto el uso de fármacos que actúan sobre mecanismos patogénicos de la enfermedad, como el efecto positivo de mejorar la calidad de vida mediante un soporte integral multidisciplinar, incluyendo nutrición, actividad física, educación, soporte emocional y paliación de síntomas. Todo ello buscando que el paciente esté lo mejor posible el mayor tiempo posible desde el diagnóstico. Pero el objetivo de las nuevas terapias anti-fibróticas combinadas que en estos momentos se evalúan en ensayos clínicos persigue aumentar el beneficio terapéutico o parar completamente la progresión de la enfermedad mediante el potencial efecto sinérgico anti-fibrótico, al actuar sobre diferentes vías patogénicas a la vez. Finalmente, se investiga qué marcadores podrían ser útiles para identificar pacientes que se puedan beneficiar más de unos fármacos anti-fibróticos u otros, lo que permitiría optimizar recursos e iniciar los primeros pasos hacia una medicina de precisión en fibrosis pulmonar. A continuación, se revisan los principales puntos de mejora en el tratamiento farmacológico de la FPI futuribles a corto, medio y largo plazo.
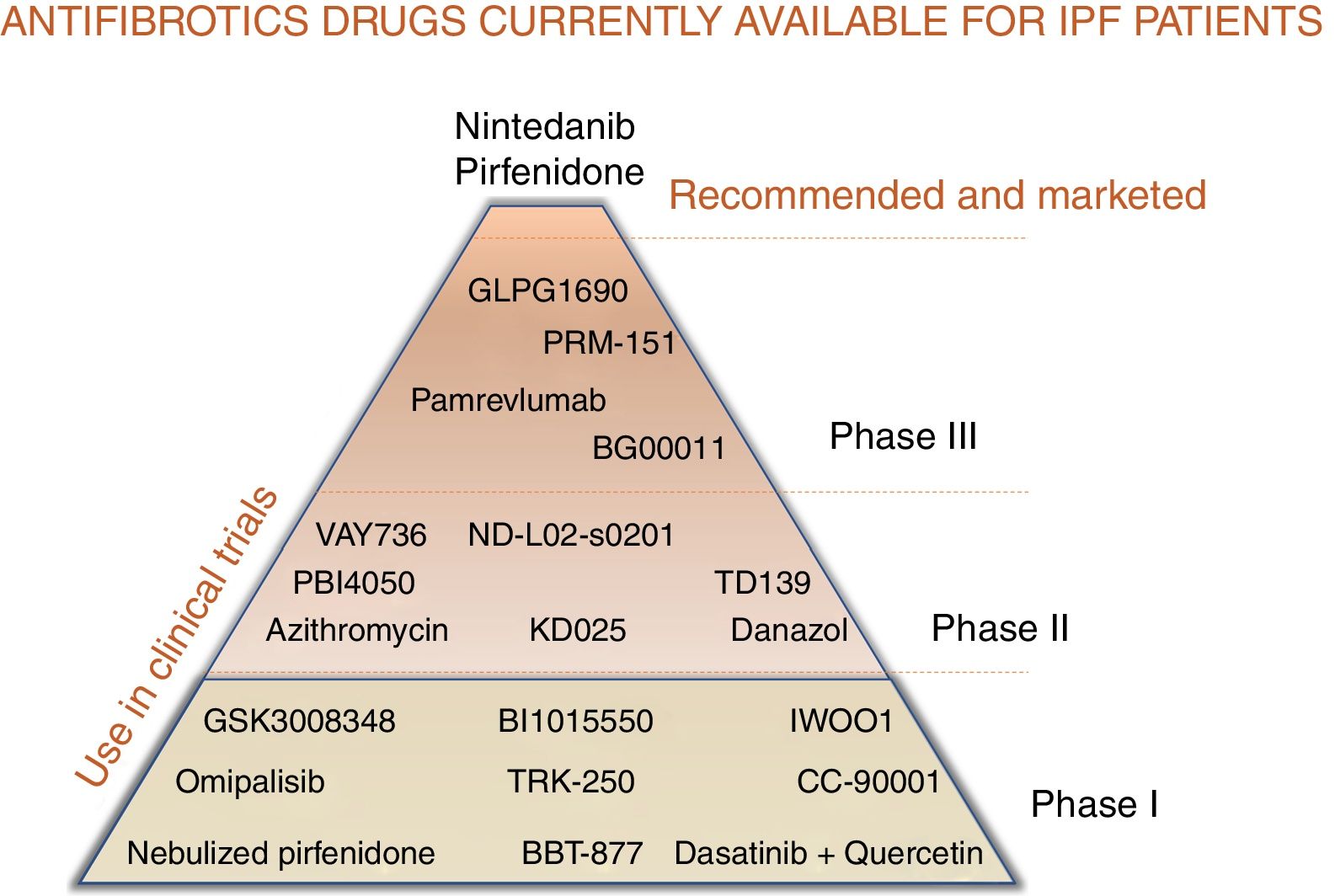
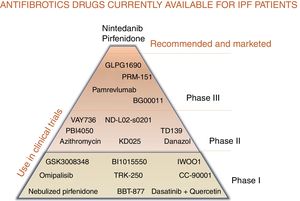
For patients with a diagnosis of idiopathic pulmonary fibrosis (IPF), 2 pharmacological options are currently available to modify the natural course of the disease: pirfenidone and nintedanib.1–9 Both delay forced vital capacity (FVC) decline and reduce the annual risk of death, and, as such, constitute a great therapeutic advance.1–9 However, the next step would be to completely arrest disease progression and make IPF no longer synonymous with deadly disease.10,11 Like neoproliferative blood diseases, such as leukemia or lymphoma, or infectious diseases, such as HIV, which also involve different pathogenic pathways, IPF can only be fully controlled by the combination of synergistic drugs acting on different mechanisms.11,12 Both pirfenidone and nintedanib are pleiotropic compounds that inhibit different profibrotic components.3–9 Experience with both drugs has taught us how to optimize adherence and reduce side effects, and the multidisciplinary approach used in most expert centers encourages patient empowerment, improves their nutrition and mental wellbeing, and promotes physical activity.13 Other antifibrotics have demonstrated their effect as inhibitors of pulmonary fibrosis in the laboratory, and their potential benefit in patients is now being evaluated in clinical trials (Table 1, Fig. 1). Moreover, not all IPF patients have the same clinical course or identical biological characteristics, so the effect of drugs can vary depending on the predominant pathogenic pathways of each case.11,12 Identifying patients who will respond better to certain drugs will help optimize treatment and improve its effectiveness.12
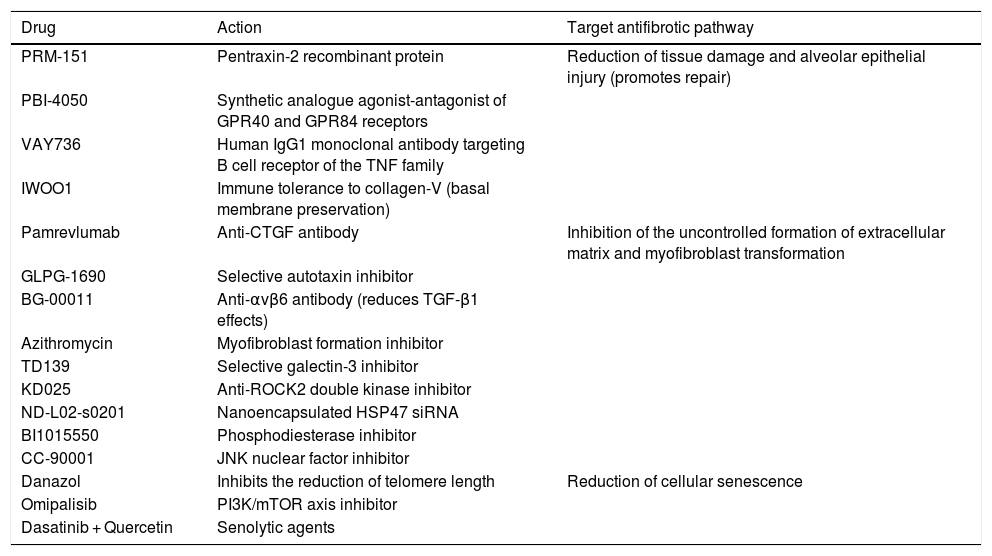
New antifibrotic drugs in clinical trials, according to therapeutic target and profibrotic mechanism of action.
| Drug | Action | Target antifibrotic pathway |
|---|---|---|
| PRM-151 | Pentraxin-2 recombinant protein | Reduction of tissue damage and alveolar epithelial injury (promotes repair) |
| PBI-4050 | Synthetic analogue agonist-antagonist of GPR40 and GPR84 receptors | |
| VAY736 | Human IgG1 monoclonal antibody targeting B cell receptor of the TNF family | |
| IWOO1 | Immune tolerance to collagen-V (basal membrane preservation) | |
| Pamrevlumab | Anti-CTGF antibody | Inhibition of the uncontrolled formation of extracellular matrix and myofibroblast transformation |
| GLPG-1690 | Selective autotaxin inhibitor | |
| BG-00011 | Anti-αvβ6 antibody (reduces TGF-β1 effects) | |
| Azithromycin | Myofibroblast formation inhibitor | |
| TD139 | Selective galectin-3 inhibitor | |
| KD025 | Anti-ROCK2 double kinase inhibitor | |
| ND-L02-s0201 | Nanoencapsulated HSP47 siRNA | |
| BI1015550 | Phosphodiesterase inhibitor | |
| CC-90001 | JNK nuclear factor inhibitor | |
| Danazol | Inhibits the reduction of telomere length | Reduction of cellular senescence |
| Omipalisib | PI3K/mTOR axis inhibitor | |
| Dasatinib + Quercetin | Senolytic agents |
Finally, developing reparative or curative treatment in pulmonary fibrosis is a long-term task, although some promising experimental results have emerged on tissue regeneration in damaged lungs14–16 (Fig. 2).
Optimization of current treatment in idiopathic pulmonary fibrosisThe treatment of the IPF patient should not be limited to prescribing nintedanib or pirfenidone.13 Therapeutic adherence and maintenance depends on several factors, including the degree of information and education that patients receive about their disease, the potential effects of the drugs, and the prevention and rapid treatment of any side effects.13 Before starting treatment, it is important to assess the patient’s disease stage, prognostic factors, and comorbidities, and their clinical and family situation.1,2 A holistic or integrated approach comprises a range of interventions, including symptomatic treatment, pharmacological treatment with antifibrotics, avoidance of factors that might aggravate the disease (especially viral infections and gastroesophageal reflux), management of comorbidities (emphysema-airway obstruction, pulmonary hypertension), physiotherapy and rehabilitation to maintain quality of life and autonomy, emotional and psychological support, and oxygen therapy when required. Lung transplantation should always be considered in cases that meet the criteria.13 Finally, in the advanced or terminal stage of the disease, the coordination of palliative treatment with home care services is essential.1,2 Ideally, IPF is managed by a multidisciplinary team that encompasses pulmonologists, pharmacists, specialist nurses, physiotherapists, rehabilitation staff, and psychologists, to address the most important needs.1,2
Pirfenidone is a drug that reduces transforming growth factor® (TGF-beta1) and platelet-derived growth factor (PDGF).1,3,4 Its efficacy has been evaluated in patients with mild-to-moderate IPF (FVC ≥ 50%, DLCO ≥ 30%–35%) in randomized, double-blind, placebo-controlled clinical trials (CAPACITY-1, CAPACITY-2, ASCEND, SP2, SP3),1,3,4 and its long-term safety has been assessed in the PASSPORT clinical trial. The most important benefits of this drug include reduced FVC decline and a significant reduction of 48% in the relative risk of death at 1 year4; these effects are independent of the initial FVC value.5 The recommended dose is 2403 mg/day, divided into 3 doses, taken with meals, which is reached over a 3-week escalation period, after performing liver panels to check tolerance.1,3,4 The most significant adverse events are gastrointestinal symptoms (nausea, dyspepsia, anorexia, and weight loss), followed by rash, photosensitivity, and to a lesser extent, liver function changes.3,4 Most of these effects can be prevented, and if they do occur, they are usually manageable and more common in the first 6 months.3,4 Phase I trials are currently assessing a new nebulized formulation of pirfenidone (100 mg) which aims to reduce side effects while achieving a greater systemic concentration of the drug (Fig. 1).
Nintedanib is a triple tyrosine kinase receptor inhibitor that targets PDGF, fibroblast growth factor (FGF) and endothelial vascular growth factor receptors.6–8 Randomized, double-blind, placebo-controlled trials support the clinical usefulness of the drug in mild-to-moderate IPF (TOMORROW, INPULSIS-1, and INPULSIS-2).6,7 Nintedanib at a dose of 150 mg/12 h inhibits FVC decline, lengthens the time until the first serious exacerbation, and reduces the risk of death at 1 year.6–8 Its beneficial effect is independent of the existence or non-existence of emphysema and initial FVC.9 Diarrhea is a common side effect (50%–60%), but it is usually mild or moderate and manageable with an astringent diet, probiotics, Saccharomyces boulardii, and occasionally, loperamide or codeine.6–8 Other less common effects are nausea and vomiting, anorexia, weight loss, and changes in hepatic function.6,7 Nintedanib is contraindicated in patients with allergic reactions to peanuts or soybeans.6,7 The most common oral anticoagulants, such as acenocumarol or warfarin, have not been tested in clinical trials and could increase the risk of bleeding.17 For patients who require anticoagulation, the use of heparins or new-generation oral anticoagulants should be evaluated.17 The long-term safety of nintedanib has been tested in a clinical trial (INPULSIS-ON).
No studies have evaluated the effect of these drugs on the function of the major pathogenic profibrotic pathways, or, consequently, patient phenotypes. However, a clinical trial (INMARK) is currently underway to assess the biological effect of nintedanib in patients with mild IPF (FVC > 80%), and to identify the type of patient who will benefit most from this treatment.18 A recent post hoc analysis of the CAPACITY-1, 2, and INPULSIS clinical trials shows that pirfenidone has the same clinical benefit in patients with and without telomere shortening,19 although rapid disease progression is more common in patients with severe shortening.
Combined antifibrotic treatment: stop progressionThe simultaneous use of different lines of treatment could act synergistically and generate a greater clinical benefit than monotherapy, given the complexity of the pathogenic mechanisms involved.10–12 Growing understanding of the pathogenesis of the disease has led to the identification of therapeutic targets for delaying (growth factors and cells) or even arresting progression by attempting to modify the altered reparative response (phenomena also seen in accelerated aging).14 The possibility of combining more than one antifibrotic drug is being investigated in currently ongoing clinical trials (Fig. 1).
To date, few clinical trials combining 2 drugs have been completed. Adding the antioxidant N-acetylcysteine to pirfenidone led to an increase in adverse gastrointestinal effects, with no increase in therapeutic benefit.20 The combined use of nintedanib with sildenafil in patients with severe IPF (DLCO <35%) also failed to show greater benefit.21 The primary objective of this clinical trial (INSTAGE) was to evaluate the effect on quality of life21 and, although the outcome was negative, the effect of both drugs on other variables is being analyzed, particularly in the subgroup of patients with greater right heart involvement. The study revealed that nintedanib treatment in severe IPF achieved rates of FVC decline (in mL) similar to mild-moderate cases in the INPULSIS study.21 The clinical trial investigating the combination of pirfenidone and nintedanib in severe IPF with pulmonary hypertension was recently completed, but the results are still pending.22 Two phase II clinical trials that combine pirfenidone and nintedanib have shown that this treatment is safe and does not increase the percentage of serious adverse effects.23,24 Furthermore, adding nintedanib to pirfenidone (INJOURNEY study) produced a non-significant increase in FVC at 8 weeks of treatment.23
Other clinical trials are ongoing with new antifibrotics to evaluate: 1) drug safety (phase I and phase II); and 2) the benefit in IPF of the administration of these compounds in monotherapy or in combination with pirfenidone or nintedanib (phase II and phase III) (Table 1, Fig. 1; https://clinicaltrials.gov). Pamrevlumab is a monoclonal antibody targeting connective tissue growth factor (anti-CTGF) that was well tolerated in a phase II clinical trial (PRAISE). The test dose was 30 mg/kg administered every 3 weeks, and results showed it to be safe in monotherapy or in combination with nintedanib or pirfenidone, with a non-significant reduction in FVC decline and loss of quality of life (USCD-SOBQ).25
GLPG1690 is a novel, orally available, selective autotaxin inhibitor which reduces cell migration and the production of tenascin-C and collagen.26 The autotaxin-lysophosphatidic phosphate phosphatase axis is increased in IPF, and GLPG1690 pleiotropically inhibits lung fibrosis in vivo and in vitro. The results of the phase II FLORA clinical trial showed that GLPG1690 (600 mg/day) is safe and is also useful for monitoring the biological effect by reducing serum concentrations of the autotaxin-lysophosphatidic acid axis.26 GLPG1690 is currently being evaluated in combination with nintedanib or pirfenidone in the phase III ISABELA clinical trial.
BG-00011 (STX-100) is a monoclonal antibody directed against the 〈v®6 integrin receptor, which blocks SMAD2 phosphorylation in bronchoalveolar lavage.27 The phase IIb clinical trial demonstrated drug safety, and BG-00011 is currently in phase III development.
PRM-151 is a recombinant form of the endogenous human protein pentraxin-2 (PTX -2), which is activated to repair tissue damage.28 This agonist regulates macrophage differentiation. In phase I and II, PRM-151 (10 mg/kg/day intravenously) has demonstrated good tolerability and safety, and the exploratory results in FVC and DLCO are encouraging.28
Azithromycin is a macrolide with immunomodulatory activity that can reduce lung damage and inhibit myofibroblast transformation.29 A phase II trial is currently ongoing to assess its effect (500 mg/day orally) on cough and lung function (FVC) (NCT02173145).
PBI-4050 is a synthetic analogue with agonist and antagonist affinity for G-protein receptors, which regulates the activity of macrophages, fibroblasts/myofibroblasts, and epithelial cells.30 A recent clinical trial has demonstrated its safety, administered once a day in 4 capsules of 200 mg, for 12 weeks.30
Galectin-3 is a circulating protein that activates macrophages and myofibroblasts. TD139 is a galectin-3 inhibitor that is administered in the form of inhaled dry powder.31 TD139 has been shown in a phase I/II clinical trial (NCT02257177) to be safe and well tolerated.
Danazol is a hormone therapy that has been used in bone marrow failure since the last century.32 An exploratory study which included 27 patients with telomere shortening and different clinical expressions of the disease showed that this drug, administered orally (800 mg/day), maintained telomere length after 24 months.32 Its effect in IPF with telomere shortening is currently being evaluated in a phase II clinical trial (NCT03312400).
KD025 (SLX-2119) is a ROCK2 inhibitor that acts on macrophage infiltration, endothelial activation, and myofibroblast differentiation.33 A phase II clinical trial (NCT02688647) is underway to assess the safety and activity of the drug, administered orally (400 mg/day) for 24 weeks.
VAY736 is an antibody that acts on the B cell receptor of the TNF family (BAFF-R), decreasing active B cells and blocking the BAFF signal.34 VAY736 is administered subcutaneously every 4 weeks (NCT03287414).
ND-L02-s0201 (BMS-986263) is a lipid nanoparticle that encapsulates an siRNA that silences the HSP47 protein (a collagen-specific chaperone associated with poor prognosis),35 which is administered intravenously (NCT03538301).
BI1015550, a new phosphodiesterase inhibitor, is being evaluated in escalating oral doses in a phase I trial (NCT03422068).
CC-90001 is a JNK inhibitor which has shown good tolerance at doses of 200 mg/day and 300 mg/day in a limited number of patients (phase Ib) after 12 weeks, showing an increase in FVC in 83% of cases, and reduced serum levels of tenascin-C (a marker of fibrotic activity) (NCT03142191).
IWOO1 is a well tolerated oral drug that prevents the degradation of collagen V in patients with positive anti-collagen V antibody, and appears to stabilize FVC (phase I trial NCT01199887).
Experimental treatments with reparative or curative intentOnce the physiological factors involved in tissue repair, which are mostly associated with the phenomena of aging, have been identified and evaluated, the next step will be to investigate treatments that can regulate these changes, with the aim of repairing injured tissue.10,11,14,36
The phosphoinositol-3-kinase (PI3K)/mTOR axis is essential for cell functions, including metabolism, division, proliferation, and autophagy, and its inhibitor, omipalisib (GSK2126458), is a potent antifibrotic (phase I).37 Increased cellular senescence is associated with the accelerated aging characteristic of IPF that underlies the development and progression of the disease.11,14 The senescence-associated secretory phenotype observed initially in alveolar epithelial cells and later in other cell strains induces collagen production and other profibrotic phenomena.38 Thus, reducing cellular senescence, both by inducing senolysis and by reversing senescence, is a new therapeutic objective.13,38 Most studies with senolytic drugs have been conducted in animal models.38 A non-randomized pilot study recently evaluated the safety of intermittent weekly senolytic treatment (dasatinib 100 mg/day + quercetin 1250 mg/day) for 3 weeks in 14 patients with IPF.39 Positive exploratory clinical and biological results have led to the development of ongoing clinical trials (Fig. 1).
Accelerated telomere shortening is a biological characteristic that can be identified in 25% of patients with IPF.13,40 Gene mutations of the enzyme telomerase are identified in a limited percentage of cases (11% in sporadic cases, 25% in family).13,40 Patients with short telomeres present higher 3-year mortality, especially when the disease develops in younger individuals (under 60 years of age); moreover, poor prognosis in these patients is independent of the type of radiological fibrosing pattern.13,40 Immunosuppressive treatment is associated with greater deleterious effects in these patients,41 a situation which is of special interest in non-idiopathic pulmonary fibrosis, where the use of immunosuppressive treatments is common. Finally, mutations in telomerase genes can be associated with other hematologic, neoproliferative, immunological, and degenerative diseases, so this therapeutic target is of interest not only in IPF, but also in other forms of telomere-driven diseases.42 Recent studies show that the regulation of telomerase reduces telomere dysfunction and DNA damage.43,44 GSE4 (gestelmir) is a dyskerin peptide that inhibits oxidation and telomere deficiencies, reduces cellular senescence phenomena, and inhibits induced pulmonary fibrosis in animal models.43 AAV9-Tert is a gene treatment that activates telomerase, retrieving the proliferative capacity of alveolar epithelial cells and decreasing bleomycin-induced pulmonary fibrogenesis.44
Thyroid hormone is critical for the maintenance of cellular homeostasis during stress responses.45 Aerosolized delivery of thyroid hormone increased survival and improved induced fibrosis in an animal model by promoting biogenesis and mitochondrial bioenergetics and reducing associated alveolar apoptosis.45
The proteasome is a multicatalytic enzyme complex (20S proteasome) that cleaves polypeptides after different amino acids, and is key for the elimination of lysis products in the reparative process.46 Oprozomib is the first oral inhibitor tested in experimental studies that reduces the pulmonary fibrogenic response.46
The WNT-®-catenin signaling pathway, critically involved in the regulation, differentiation, proliferation and cell death, is altered in pulmonary fibrosis, thus affecting reparative development.47 Direct and indirect regulation of this cascade by altering exosome activity has been investigated in the laboratory.48
Several studies have tested mesenchymal stem cells and other pluripotent cells as possible antifibrotic treatment, given their immunomodulatory, anti-inflammatory, and antifibrotic potential.49 The function of these cells depends on different factors, including primarily age and the existence of a biological reparative basis, such as occurs in IPF.49 Therefore, most studies have used allogeneic cell transplantation.16 Different trials have evaluated the intravenous or endotracheal administration of various pluripotent cells of varying degrees of purity and different origins (bone marrow, fat, umbilical cord, lung), and different times and pretreatments, but, for the moment, although it seems a safe therapy, none of the studies could be replicated in multicenter studies with parallel control groups.16,50 The most significant limitations include difficulties in isolating cells and achieving purity, the half-life of the pluripotent cells after infusion, and poor control of cell cycle phases.16,49 We are gradually overcoming all these difficulties51,52: some methods use less lung tissue to obtain lung spheroid cells51; original cells with greater reparative power are selected; and the therapy is simplified, focusing on a benefit from the antifibrotic and reparative effect of the products of these cells (growth factors such as HGF and microvesicles).52
ConclusionMore than 200 antifibrotic drugs have been developed experimentally; most do not reach the clinic or do not succeed in trials. Today, however, in addition to the 2 antifibrotic drugs in monotherapy, 4 more drugs in phase III clinical trial are being evaluated in combination with pirfenidone or nintedanib in an attempt to improve control or to stop progression. In the long term, we might be able to prescribe individualized combined therapies and repair lung damage.
FundingInstituto de Salud Carlos III (ISCIII) PI18/00367, co-funded by European Regional Development Funds (ERDF: a way to build Europe).
Conflict of interestsThe research group led by the author receives or has received funding in the form of grants or payment for the provision of scientific services from Roche, Boeringher Ing., Chiesi, Esteve Teijin Healthcare, Linde, GSK, Astra-Zeneca, Intermune.
Please cite this article as: Molina-Molina M. Futuro del tratamiento farmacológico en fibrosis pulmonar idiopática. Arch Bronconeumol. 2019;55:642–647.