Two studies published in the early 80s, namely the Nocturnal Oxygen Therapy Trial (NOTT) and the Medical Research Council Trial (MRC), laid the foundations for modern home oxygen therapy. Since then, little progress has been made in terms of therapeutic indications, and several prescription-associated problems have come to light. Advances in technology have gone hand in hand with growing disregard for the recommendations in clinical guidelines on oxygen therapy. The introduction of liquid oxygen brought with it a number of technical problems, clinical problems related to selecting candidate patients for portable delivery devices, and economic problems associated with the rising cost of the therapy. Continuous home oxygen therapy has been further complicated by the recent introduction of portable oxygen concentrators and the development in quick succession of a range of delivery devices with different levels of efficiency and performance. Modern oxygen therapy demands that clinicians evaluate the level of mobility of their patients and the mobility permitted by available oxygen sources, correctly match patients with the most appropriate oxygen source and adjust the therapy accordingly. The future of continuous home oxygen therapy lies in developing the ideal delivery device, improving the regulations systems and information channels, raise patient awareness and drive research.
Dos estudios publicados a comienzo de los 80, el Nocturnal Oxygen Therapy Trial (NOTT) y el Medical Research Council Trial (MRC), sentaron las bases de la oxigenoterapia domiciliaria moderna. Desde entonces, se ha avanzado muy poco en el campo de las indicaciones mientras que se han puesto en evidencia múltiples problemas relacionados con su prescripción. La falta de adherencia a las recomendaciones establecidas por las guías clínicas en oxigenoterapia es un fenómeno que ha ido aumentando en paralelo al desarrollo tecnológico. La incorporación del oxígeno líquido trajo de la mano problemas técnicos, problemas clínicos relacionados con la selección de pacientes candidatos a beneficiarse de equipos portátiles y problemas económicos, al aumentar el coste de la terapia. La incorporación reciente de los concentradores portátiles ha complicado aún más la oxigenoterapia continua domiciliaria poniendo a nuestra disposición equipos de oxígeno con rendimientos y prestaciones muy variables a la velocidad de la innovación tecnológica. La oxigenoterapia moderna exige tener que valorar el perfil de movilidad de los pacientes, la movilidad permitida por las fuentes de oxígeno disponibles, la adecuada selección de paciente y fuente de oxígeno y la necesidad de titular la oxigenoterapia. La búsqueda del equipo de oxígeno ideal, mejorar los sistemas de regulación e información de las terapias, avanzar en la educación de los pacientes y potenciar la investigación, son líneas de trabajo que nos marcan el futuro de la oxigenoterapia continua domiciliaria.
Oxygen has been used for medicinal purposes almost since its discovery by Joseph Priestley in 1772.1,2 After a period in which it was used for virtually everything (with varying results), Alvin Barach was the first to use it rationally, demonstrating its effectiveness in cor pulmonale and acute and chronic respiratory failure. Considered the father of modern oxygen therapy,3 he led the way in ambulatory oxygen therapy, with the development of the first portable devices and oxygen-conserving systems.
In the early 80s, 2 studies were conducted which, despite their limitations, laid the foundations for long-term home oxygen therapy (LTOT) as we know it today: the Nocturnal Oxygen Therapy Trial (NOTT) and the Medical Research Council Trial (MRC)4,5 (Table 1). The legacy of these studies was the recommendation that LTOT should be prescribed to patients with chronic obstructive pulmonary disease (COPD) who had stopped smoking, were receiving optimal medical treatment and who, when clinically stable, had sea level PaO2 values of less than 55mmHg or between 55 and 60mmHg if there was evidence of damage due to hypoxia (arrhythmias, cor pulmonale, right heart failure, polycythemia or impaired intellectual function). These criteria for prescribing long-term oxygen therapy have been included since then in all LTOT guidelines, and have also appeared in the recently published SEPAR guidelines on “Continuous Home Oxygen Therapy”6 and in the recommendations for prescribing home respiratory therapies7 (Table 2). In these patients, the use of oxygen for at least 15h a day improves survival and quality of life. Long-term oxygen therapy also reduces right heart failure caused by cor pulmonale, improves neuropsychological function, and increases exercise tolerance and the ability to perform activities of daily living.6
NOTT and MRC Trials Inclusion Criteria and Patients.
| NOTT (1980)4 | MRC (1981)5 | |
| Patients | 203 patients (77% M)101 with continuous O2 (24h/d)/102 with nocturnal O2 (12h/d) | 87 patients (75% M)LTOT (15h/d) vs no LTOT |
| Inclusion criteria | Clinical diagnosis of COPDAge >35 yearsPaO2 <55mmHg or PaO2 <59mmHg plus one of the following:– Edema– Hematocrit >55%– Cor pulmonale on ECG– FEV1/FVC <70%– TLC >80% | Age <70 years (42–69)FEV1 <1.2lPaO2 between 40 and 60mmHg in 2 tests performed 3 weeks apart |
ECG: electrocardiogram; COPD: chronic obstructive pulmonary disease; FEV1/FVC: forced expiratory volume in the first second divided by forced vital capacity; h/d: hours/day; MRC: Medical Research Council Trial; NOTT: Nocturnal Oxygen Therapy Trial; O2: oxygen; LTOT: long-term home oxygen therapy; PaO2: partial oxygen pressure in arterial blood; TLC: total lung capacity; M: males.
Current Guidelines on Long-Term Home Oxygen Therapy.
| Guideline | Severe hypoxemia | Moderate hypoxemia | No hypoxemia |
| Ministry of Health and Long-Term Care48 | PaO2 <55mmHg or Sat <88% | PaO2 56–60mmHg plus: cor pulmonale, pulmonary hypertension, polycythemia, exercise limited by hypoxemia that improves with O2 or nocturnal hypoxemia | Patients with exercise-induced hypoxemia who improve exercise tolerance with O2 |
| ATS-ERS49 | PaO2 <55mmHg or Sat <88% | PaO2 55–59mmHg plus: cor pulmonale, edema or hematocrit >55% | PaO2 >60mmHg or Sat >90% with severe nocturnal desaturation and dyspnea of respiratory origin that improves with O2 |
| GOLD50 | PaO2 <55mmHg or Sat <88% with or without hypercapnia | PaO2 56–59mmHg plus: cor pulmonale, edema or hematocrit >55% | No recommendation |
| NICE51 | PaO2 <55mmHg | PaO2 56–59mmHg plus: polycythemia, nocturnal hypoxemia (SpO2 <90% more than 30% of recording time) or peripheral edema | No recommendation |
| TSA-NZ52 | PaO2 <55mmHg | PaO2 56–59mmHg plus: evidence of damage due to hypoxia including right heart failure, pulmonary hypertension or polycythemia | Nocturnal O2 indicated if SpO2 <88% for more than 30% of nighttime recording or hypoxia-related sequelae |
| AIPO53 | PaO2 <55mmHg | PaO2 55–60mmHg plus: hematocrit >55%, pulmonary hypertension, edema, right heart failure, mental disturbance or ischemic heart disease | Intermittent O2 indicated if SpO2 <90% for more than 30% of the nocturnal recording or desaturation on exercise |
| SEPAR6 | PaO2 <55mmHg | PaO2 56–59mmHg with evidence of organic damage due to hypoxia (including right heart failure, pulmonary hypertension or polycythemia) | Desaturation on exercise (SpO2 <88%) that improves with O2Nocturnal desaturation (SpO2 <90% for at least 30% of the total recording time) and hypoxia-related sequelae |
AIPO: Italian Association of Hospital Pulmonologists; ATS-ERS: American Thoracic Society and European Respiratory Society; GOLD: Global Initiative for Chronic Obstructive Lung Disease; mmHg: millimeters of mercury; NICE: National Institute for Health and Clinical Excellence; PaO2: partial oxygen pressure in arterial blood; SEPAR: Spanish Society of Pulmonology and Thoracic Surgery; SpO2: O2 saturation by pulse oximetry; TSA-NZ: Thoracic Society of Australia and New Zealand.
Modification of COPD Working Group. Long-term oxygen therapy for patients with chronic obstructive pulmonary disease (COPD).54
It is essential to understand that oxygen administration only corrects hypoxemia during application, and has no residual effect. When the supplemental oxygen supply is discontinued, hypoxemia reappears; oxygen therapy must therefore be maintained for at least 15h per day in order for it to be beneficial.6 It is equally important to bear in mind that the indication for prescribing long-term oxygen therapy in patients with severe hypoxemia was established because of its effect on survival.3,4 In all cases, LTOT should be considered after confirming respiratory failure in 2 separate blood gas measurements taken 3 weeks apart, in a clinically stable phase, after an exacerbation-free period of at least 3 months.6 The oxygen flow prescribed should be sufficient to achieve a PaO2 of over 60mmHg or 90% arterial oxygen saturation measured by pulse oximetry (SpO2), without this triggering acute hypercapnia or acidosis.6 Finally, LTOT should be reconsidered in patients who, despite meeting the necessary requirements, continue to smoke, have a clear history of poor treatment compliance or are unable to correctly handle oxygen supply systems.6
After the Nocturnal Oxygen Therapy Trial and the Medical Research Council Trial, What Next?The criteria established by the NOTT and MRC trials have remained consistent for more than 30 years, despite advances in the treatment of respiratory diseases, the introduction of home non-invasive mechanical ventilation, identification of patients with sleep apnea syndrome, and improved phenotypic differentiation of COPD patients, all of which are confounding factors in patients with chronic respiratory failure. That these recommendations have remained unchanged has mainly been due to the lack of studies in LTOT during this time.8 Feeble attempts have been made to assess the efficacy of oxygen therapy in patients with moderate hypoxemia (PaO2 55–65mmHg), such as the studies by Gorecka et al.9 and Haidl et al.,10 neither of which were able to demonstrate improved survival. These studies were methodologically very limited and provided no information on the effect of oxygen on other clinical variables, such as dyspnea or quality of life. In this regard, a recent meta-analysis11 selecting very heterogeneous studies found that oxygen therapy significantly improves dyspnea. Another study, likewise based on incomplete data, showed that LTOT could improve health-related quality of life, depression and cognitive function in patients, and could have a positive impact on the frequency of hospitalization.12 These data suggest that if we focus on survival alone, we could be overlooking clinical variables on which LTOT could have a positive effect, and suggests the need for studies that more clearly define the objectives to be reached when prescribing oxygen therapy.
Other situations that have been cautiously explored by the scientific community concern the use of oxygen in patients with isolated exertional or nocturnal oxygen desaturation. Although oxygen therapy during exercise may increase exercise tolerance and reduce dyspnea, it has not been shown to have any impact on survival.13,14 In this uncertain setting, SEPAR guidelines recommend the use of oxygen during exercise in these patients if there is evidence that it corrects hypoxemia and improves dyspnea or exercise tolerance, increasing the distance covered in the 6-min walk test by at least 25–30m.6,15–17 These guidelines also consider the administration of oxygen to patients with exercise-induced desaturation enrolled in rehabilitation programs, in order to increase the duration and intensity of training.6,18
There is also little information on the effect of oxygen therapy in patients with nocturnal hypoxemia without daytime respiratory failure. Nocturnal hypoxemia has been arbitrarily defined as an SpO2 <90% for more than 30% of the night,19 although some authors have used more flexible criteria.20 Current evidence suggests that nocturnal oxygen therapy does not improve survival in COPD patients with nocturnal desaturation alone.21 SEPAR guidelines recommend (with a low level of evidence) the use of nocturnal oxygen therapy in patients with nocturnal desaturation (SpO2 <90% for more than 30% of the recording time) and clinical consequences due to hypoxia (polycythemia or signs of right heart failure).6
Problems Associated With Long-Term Home Oxygen TherapyAs we have seen, little progress has been made in the indications for LTOT since the publication of the NOTT and MRC trials, but what has become evident, however, are innumerable prescription-related problems: its use has been extended to non-COPD patients with respiratory failure; the need to titrate the oxygen flow has been demonstrated, while it is a fact that in many patients instead of significantly improving hypoxemia, we instead worsen their clinical condition22,23; a high rate of treatment non-compliance has been found24; oxygen therapy is being maintained in patients who continue to smoke25; LTOT is being prescribed in subjects who are not clinically stable, and maintained after they have recovered; oxygen is being used for symptomatic treatment of dyspnea and for palliative purposes in patients with terminal cancer, when the superiority of opiates has been demonstrated in these situations26; oxygen is being combined with other therapies such as nocturnal continuous positive airway pressure (CPAP) or non-invasive mechanical ventilation, even though the benefits of this are so far unproven,27 and many prescriptions are written using sea level criteria, regardless of the altitude of the patient's home.28
In short, prescription and administration of LTOT have changed far beyond the recommendations set out in clinical guidelines, and these are routinely disregarded. Some studies have also tried to differentiate continuous (LTOT) from short-burst oxygen therapy for the relief of dyspnea, where it has a significant placebo effect,29 giving the impression that oxygen can be used for anything, anyone and anywhere, all of which causes constant friction between pulmonologists, patients and family members when we try to rationalize the use of oxygen. The situation is made worse by the fact that supply depends on different companies, according to where the patient lives. This creates problems in the home maintenance of equipment and consumables, and makes patient mobility (and ultimately treatment compliance) more complex.30
A New Problem: Liquid OxygenThe Fifth Oxygen Consensus Conference held in Denver in 198731 was a milestone in the history of LTOT insofar as it called for oxygen to be used both inside and outside the home by patients capable of living an active life, consolidating the concept of ambulatory oxygen therapy. Oxygen equipment for such purposes must be light, with enough autonomy to guarantee the patient adequate oxygen supply while engaged in activities away from home. After a period where small bottles of compressed oxygen were the only option for receiving oxygen outside the home, liquid oxygen was introduced as an attractive alternative to conventional LTOT.32 This new scenario brought with it new problems: technical difficulties related to oxygen sources, clinical problems associated with the selection of patients who could benefit from portable oxygen equipment and, obviously, economic problems due to the rising costs of therapy. The Spanish Ministry of Health and Consumer Affairs, in an Order dated 3 March 1999, established that oxygen therapy using liquid oxygen sources could only be prescribed in patients who required flow rates greater than 5l/min, who were able to work or walk, and who could not do so without an oxygen supply.33 To check the efficacy of this particular therapy, the Order required that the patient perform three 6-min walk tests (four if a practice walk was also done) with and without oxygen (baseline, titrated and response evaluation) to demonstrate improved exercise tolerance.
The prescription of liquid oxygen has further complicated LTOT: there is no evidence of its efficacy34; the rates of treatment non-compliance are, as with conventional LTOT, very high35,36; failure to accurately titrate the oxygen flow required to correct hypoxemia means that patients remain desaturated on exertion despite receiving oxygen; it is prescribed to all types of patients without discriminating between COPD and other diseases, including cancer; and the walk test is perfunctory, meaning that liquid oxygen generally recommended based on the subjective criteria of doctors, patients and family members. Additionally, the 6-min walk test is not as simple as it sometimes appears, and merely walking in front of a professional in a white coat is not a walk test.37 In order for it to be a valid measuring instrument, it must be strictly structured according to current standards,38 and implementation is erratic. In short, if our use of conventional LTOT has been poor, the introduction of liquid oxygen has further aggravated an already chaotic approach to oxygen therapy.
The appearance of liquid oxygen also had other consequences: in order to increase the autonomy of portable equipment, oxygen conservation systems were incorporated, such as the transtracheal catheter, inspiratory demand valves and expiratory reservoirs, making the world of LTOT a little more complex.39
Yet More Problems: The Portable ConcentratorTo further complicate the situation, portable oxygen concentrators (POC) have recently appeared on the market.40 These are more likely to be used outside the home, as they rely on batteries and electrical power supply, but most of those currently available release the oxygen in boluses or pulses, with pulsed-dose flows that have yet to demonstrate their efficacy compared to continuous flows.41 Furthermore, they cannot be used together with pressure equipment (CPAP, double pressure ventilators) and their nighttime use is not recommended, although some newly launched devices include this indication.42,43 This creates a situation in which patients must have various oxygen sources at home: the fixed concentrator, a bottle of compressed oxygen in case of a power cut, and a portable concentrator for outside use, which complicates LTOT even further.44 Titration problems are compounded, and no improvement in treatment adherence has been found either. Finally, home respiratory therapy suppliers are now faced with escalating costs and accordingly indications are more restrictive, as reflected in the agreements for home-based therapies made with health authorities.7
One characteristic inherent to oxygen therapy using POC is that it is highly dependent on technological innovation. Various manufacturers release increasingly sophisticated models before their efficacy has been compared with previous models (Table 3). This is a hugely important issue. Chatburn et al.41 analyzed the performance of 4 POCs (Invacare XPO[2]®, Philips-Respironics EverGo®, AirSep FreeStyle® and Inogen One®), and found major differences between them in relation to the oxygen volume per pulse, pulse duration, sensitivity of the POC trigger, and the concentration of oxygen delivered to the patient. These results emphasize the fact that each patient's POC prescription must be checked to ensure that it meets their needs both during rest and activity, and POC settings must be accurately adjusted (in this context, prescribing oxygen at 2l/min is incorrect, for example). It is important to know that each POC has different possible settings (e.g. FreeStyle® from 1 to 3, Inogen One G2® from 1 to 5, Evergo® from 1 to 6) and that each of these releases different volumes of oxygen, according to the manufacturer's criteria; therefore, titration has become an essential aspect of this therapy. It is also essential to bear in mind that POCs depend on the patient's respiratory rate (very important when it comes to use during exercise) and on nasal respiration (crucial for activating the trigger). In some POCs, the volume of oxygen delivered in each pulse decreases as the respiratory rate increases. These are POCs that operate with a fixed volume of oxygen per minute (ml O2/min), as is the case of the Inogen One®. Other POCs can maintain a constant pulse volume when the respiratory rate increases. In this case, the volume of oxygen per minute varies according to this rate; one such POC is the FreeStyle®. The Philips-Respironics Evergo® is a combination of both types. At settings 1 and 2, the pulse volume remains constant, at setting 3 it continues to remain constant if the respiratory rate is less than 25breaths/min, and at settings 4, 5, and 6, the volume reduces as the rate increases. The Inogen One® at setting 5 can deliver 750ml O2/min, which means that at 15breaths/min, the patient receives 50ml of oxygen in each bolus (manufacturer's data). The Evergo® can deliver a maximum of 1050ml O2/min, and at setting 6 can deliver 70ml oxygen in each bolus at 15breaths/min (manufacturer's data). Some POCs include a continuous flow option, as is the case of the Philips-Respironics SimplyGo®, which can be set at up to 2breaths/min and includes a sleep mode in which the apparatus switches automatically to continuous mode if no breathing is detected.
Characteristics and Performance of the Main Portable Concentrators Currently on the Market.
| Invacare SOLO2 | SeQual Eclipse 3 | OxLife Independence | DeVilbis iGo | Respironics SimplyGo | Invacare XPO2 | Inogen One G2 | Respironics EverGo | |
| Continuous and pulsed-flow | Continuous0.5–3 LPMPulse settings: 1–5 | Continuous0.5–3 LPMPulse settings: 1–9 | Continuous1–3 LPMPulse settings: 0.5–6 | Continuous1–3 LPMPulse settings: 1–6 | Continuous0.5–2 LPMPulse settings: 1–6 | ContinuousN/APulse settings: 1–5 | ContinuousN/APulse settings: 1–5 | ContinuousN/APulse settings: 1–6 |
| O2 pulse delivered at setting 2 | 32ml | 32ml | 32ml | 28ml | 24ml | 23ml | 18ml | 23ml |
| O2 pulse delivered at the maximum POC setting | 89ml | 96ml | 96ml | 84ml | 72ml | 42ml | 45ml | 52ml |
| Battery duration at setting 2 | 4.2h | 5.2h | 5.75h | 4.7h | 3.7h | 2.5h | 8h | 4h |
POC: portable oxygen concentrator; LPM: liters per minute; ml: milliliters; N/A: not applicable.
POC with continuous and pulsed-flow POC with pulsed-flow.
As we can see, POCs are sufficiently complex to merit greater attention than is currently given when we prescribe oxygen therapy. SEPAR guidelines on LTOT state that the use of POC should be restricted to patients who require low oxygen flows (<3l/min), and its efficacy should always be confirmed with an exercise test6 (Table 4).
Advantages and Disadvantages of the Main O2 Sources.
| Compressed gas cylinder | Portable gas cylinder | Portable concentrator | Liquid oxygen | |
| Advantages | Silent | Limited mobility outside the home | Unlimited autonomy provided there is access to electricity supply and/or batteries | Autonomy limited by the charge in the backpackRechargeable from supply sourceIndicated if flows >3l/min are required |
| Disadvantages | Requires distribution networkStationary sourceDoes not permit mobility | WeightRequires distribution networkPoor autonomyNon-rechargeable | WeightLoss of efficacy with high flow rates and changes in respiratory rateNot indicated if flows >3l/min are requiredNoisy | Requires distribution networkWeight |
| Cost/usefulness ratio | Low | High | High |
l/min: liters/minute.
Two questions that often arise in relation to LTOT are: Should a clinical trial be conducted to confirm the findings of 30 years ago? Would ethics committees agree to clinical trials on oxygen therapy? The NOTT4 and MRC5 trials included only 290 patients, and were conducted in the 80s. In the NOTT trial, the main inclusion criterion was an FEV1/FVC <0.7, and in the MRC an FEV1 <1.2l. Furthermore, the latter study had an age limit of 70 years. Today, the inclusion criteria for a similar study would be completely different. Since then, treatment of COPD has advanced considerably, and we all assume that the survival and quality of life of our patients are better today than it was 40 years ago.45 Our cases are nothing like those in the NOTT/MRC studies.
Increased awareness among healthcare professionals of other issues, such as sleep apnea (COPD patients may have concomitant CPAP treatment) and obesity hypoventilation (classic “Blue Bloater” patients, today treated with non-invasive ventilation with or without added oxygen), has changed our therapeutic approach to these patients. The description of phenotypes, the role of obesity and comorbidities, better understanding of bronchiectasia, inhaled antibiotic therapy, chronic bacterial infection, gastroesophageal reflux, etc. lead clinicians to wonder every day and with every patient whether oxygen plays the same role as it did in the 80s.
That said, it is not surprising that studies in asthma and COPD are continually being designed to gain greater insight into these diseases and improve treatment. What is surprising though, is that in the twenty-first century, no new and improved studies have been conducted to improve our knowledge of LTOT and better define indications or identify sub-groups of patients who would benefit more from LTOT in terms of survival, dyspnea, exercise capacity, quality of life or exacerbations. Will LTOT one day have different indications for different types of COPD? We believe that there is an undeniable need for studies in the field of LTOT that answer these and other questions.
What Can Be Done?It is important to simplify the current situation of LTOT. Manufacturers must work on developing the ideal oxygen system: a single device that can satisfy the patient's oxygen needs at any time of the day or night, inside or outside the home: so-called “deliveryless technology”.46 At present, portable concentrators and stationary concentrators that can be used to refill bottles with gaseous oxygen (e.g. the Philips-Respironics UltraFill® system) or liquid oxygen (e.g. the VIAspire® Liquefier system from Inspire Technologies)46 fall within this category. New devices must meet safety and efficacy requirements, and must have been clinically tested.
A second vitally important aspect is correct selection of patients who are likely to benefit from portable oxygen, choosing the most appropriate oxygen source for each case based on the patient's particular mobility profile and the mobility permitted by each source.44 We must also understand that oxygen therapy should be titrated and the oxygen flow adjusted to ensure correction of hypoxemia.
Thirdly, it is crucial to establish mechanisms for regulating and supervising home respiratory therapies, create centralized information systems, and have access to data on patient compliance when poor treatment adherence warnings are detected, allowing us to take decisions at the right time.
Health professionals must be given a larger role in educating patients on oxygen therapy, and these concepts should be included in COPD self-management and comprehensive care programs. Home care programs have been shown to effectively improve adherence to LTOT, and should be standard practice.47
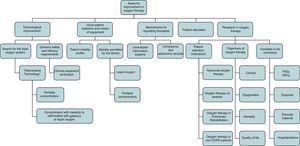
Finally, studies to define the profile of patients who would really benefit from LTOT and the variables to be monitored should be designed, and strict indication criteria should be implemented (Fig. 1). Above all, however, LTOT must be given the recognition and importance it deserves, and no longer be used as routine treatment for any type of disease or situation. In short, we need to become aware of the current chaotic situation of LTOT, raise awareness of all the issues involved, and promote quality research in LTOT. It is time to stop talking about NOTT and MRC.
Conflict of InterestThe authors declare that they have no conflict of interest.
Please cite this article as: Díaz Lobato S, García González JL, Mayoralas Alises S. Controversias en oxigenoterapia continua domiciliaria. Arch Bronconeumol. 2015;51:31–37.