Azygos vein thrombosis (AVT) and hemiazygos vein thrombosis (HVT) are rare entities. The etiopathogenesis is unknown, but these thromboses have been associated with predisposing factors, such as underlying azygos vein aneurysm (AVA)1 or other prothrombotic factors. AVT in a normal azygos and hemiazygos vein is exceptional.2 The azygos system provides the superior (SVC) and inferior vena cava (IVC) with an alternative access to the right atrium, so AVT is usually asymptomatic, making it difficult to diagnose and treat early. It can be complicated by pulmonary embolism (PE), vena cava syndrome (VCS) and even stroke in the case of cardiac shunt, which confers a worse prognosis.3 This paper reports an acute, symptomatic case of AVT and HVT and reviews the most relevant aspects of this rare entity.
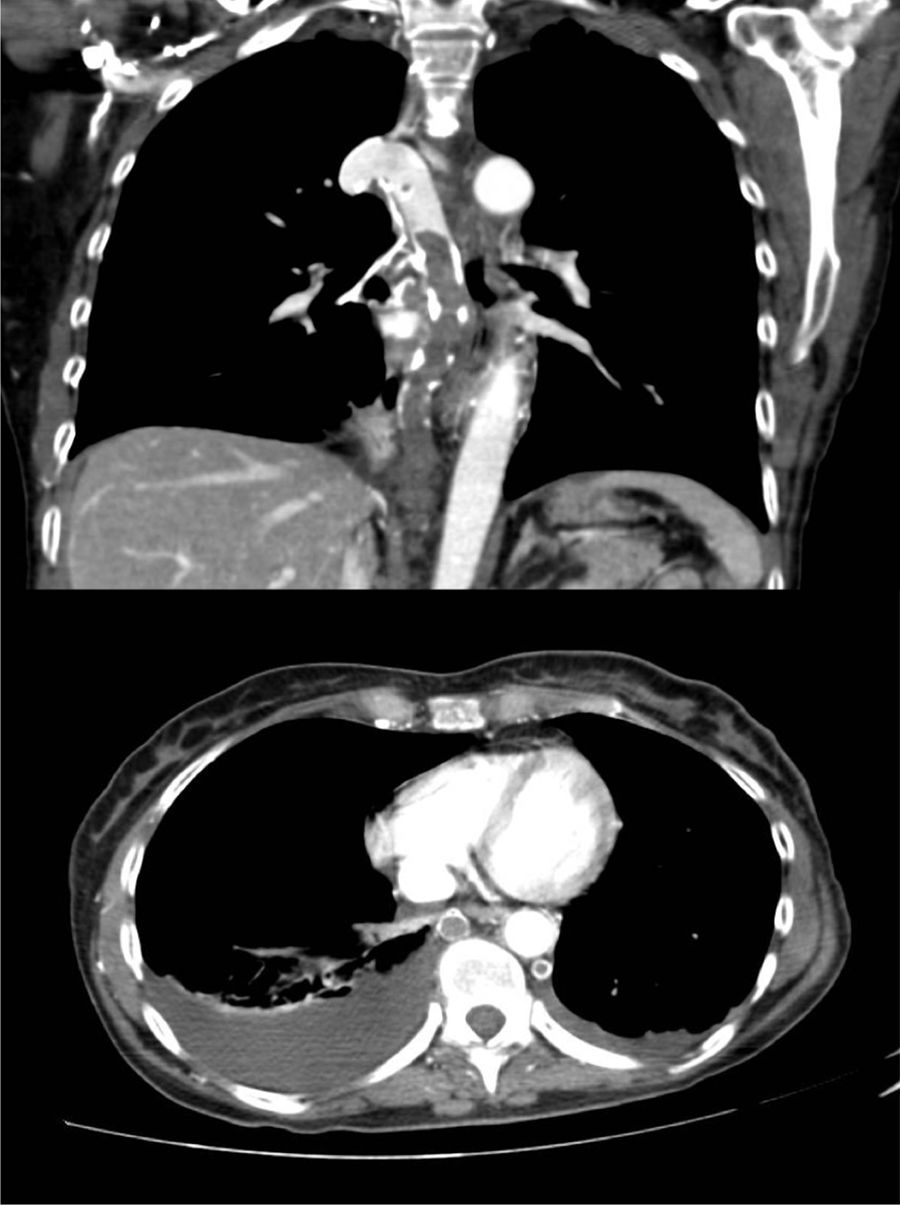
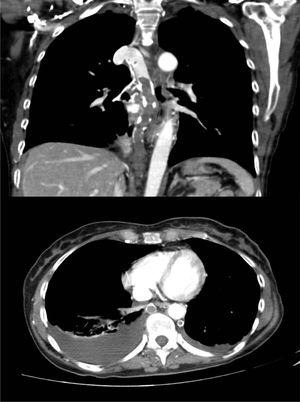
A 60-year-old woman, former smoker, receiving chemotherapy for a diagnosis of ovarian adenocarcinoma, presented with sudden dyspnea, heaviness in the upper limbs, and syncope. Physical examination revealed blood pressure 110/60 mmHg, heart rate 110 bpm, oxygen saturation 95%, erythema and facial edema, and no signs of collateral circulation or lymphadenopathies. On laboratory tests, hemoglobin was only 8.6 g/dl. Chest CT-angiogram showed extensive thromboses in the azygos and hemiazygos veins, with no signs of PE or VCS (Fig. 1). No findings of interest were observed on lower limb ultrasound or echocardiogram. Thrombophilia tests, including antiphospholipid antibodies, were negative. The patient was given weight-adjusted tinzaparin treatment and anticoagulation continued until the end of chemotherapy (9 months), with complete resolution of her respiratory symptoms and no evidence of complications. Repermeabilization of the AVT was observed in the follow-up CT.
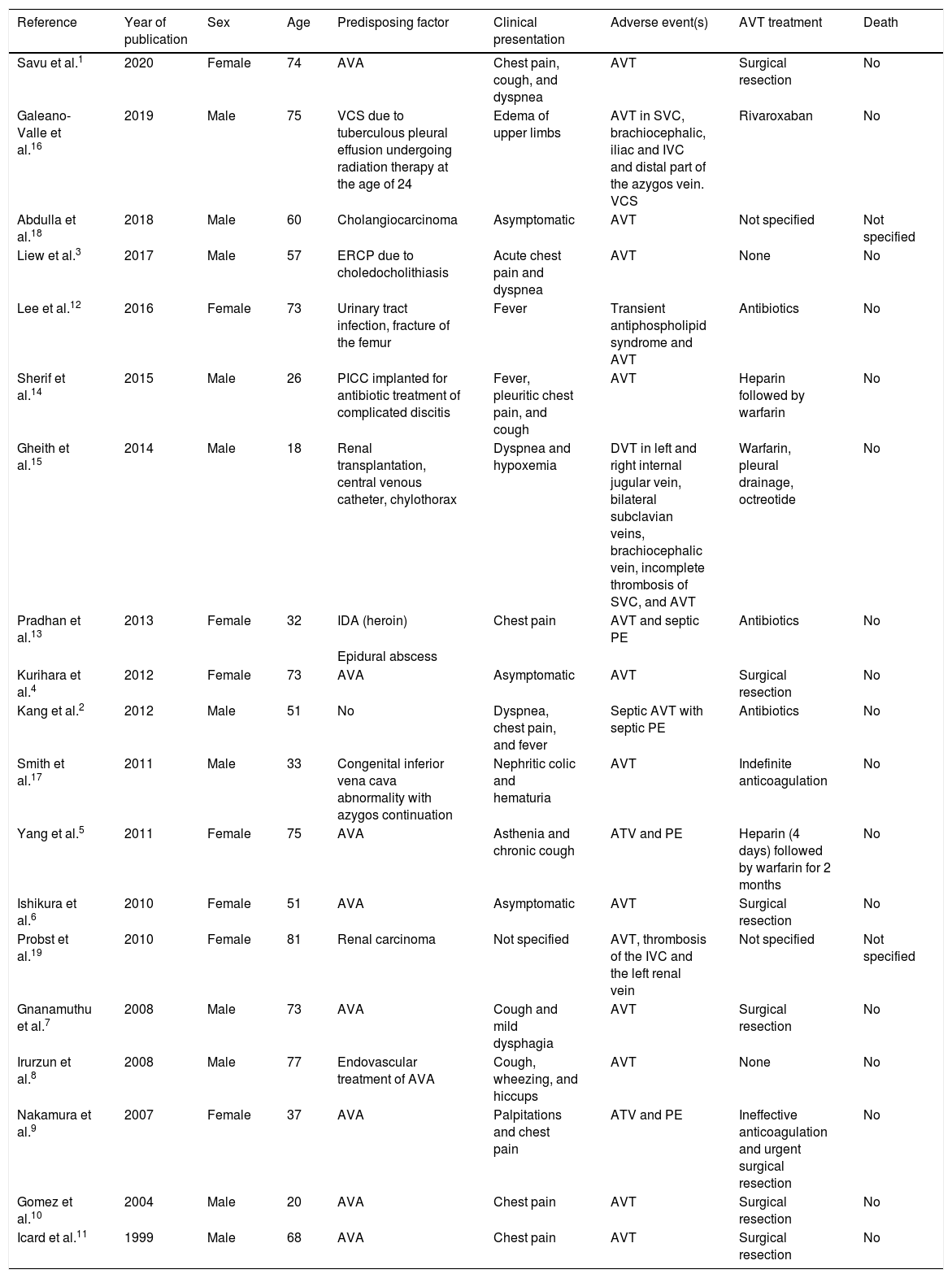
We performed a Pubmed search for original and review articles using the term “azygos vein thrombosis”. Original articles were eligible for inclusion if AVT and/or HVT were identified. Abstracts, animal studies, and articles written in languages other than English or Spanish were excluded. The references for each article included in this review were also analyzed to identify other studies of interest. We found a total of 216 eligible studies, published up to June 2020. Nineteen studies met the inclusion criteria and all reported isolated clinical cases (Table 1). Of the 19 patients included, male sex was slightly more predominant (57.8%) and the mean age was 55 years. AVA was the most frequent predisposing factor in 47.3% of cases,1,4–11 followed by septic thrombosis (15.78%)2,12,13 and intravenous catheters (10.5%).14,15 Other etiologies included other prothrombotic factors3,16 and congenital malformations.17 Although the high prevalence of venous thromboembolism (VTE) in cancer patients is well known, AVT associated with cancer is unusual.18,19
Predisposing factors, clinical presentation and treatment in studies on azygos vein thrombosis.
| Reference | Year of publication | Sex | Age | Predisposing factor | Clinical presentation | Adverse event(s) | AVT treatment | Death |
|---|---|---|---|---|---|---|---|---|
| Savu et al.1 | 2020 | Female | 74 | AVA | Chest pain, cough, and dyspnea | AVT | Surgical resection | No |
| Galeano-Valle et al.16 | 2019 | Male | 75 | VCS due to tuberculous pleural effusion undergoing radiation therapy at the age of 24 | Edema of upper limbs | AVT in SVC, brachiocephalic, iliac and IVC and distal part of the azygos vein. VCS | Rivaroxaban | No |
| Abdulla et al.18 | 2018 | Male | 60 | Cholangiocarcinoma | Asymptomatic | AVT | Not specified | Not specified |
| Liew et al.3 | 2017 | Male | 57 | ERCP due to choledocholithiasis | Acute chest pain and dyspnea | AVT | None | No |
| Lee et al.12 | 2016 | Female | 73 | Urinary tract infection, fracture of the femur | Fever | Transient antiphospholipid syndrome and AVT | Antibiotics | No |
| Sherif et al.14 | 2015 | Male | 26 | PICC implanted for antibiotic treatment of complicated discitis | Fever, pleuritic chest pain, and cough | AVT | Heparin followed by warfarin | No |
| Gheith et al.15 | 2014 | Male | 18 | Renal transplantation, central venous catheter, chylothorax | Dyspnea and hypoxemia | DVT in left and right internal jugular vein, bilateral subclavian veins, brachiocephalic vein, incomplete thrombosis of SVC, and AVT | Warfarin, pleural drainage, octreotide | No |
| Pradhan et al.13 | 2013 | Female | 32 | IDA (heroin) | Chest pain | AVT and septic PE | Antibiotics | No |
| Epidural abscess | ||||||||
| Kurihara et al.4 | 2012 | Female | 73 | AVA | Asymptomatic | AVT | Surgical resection | No |
| Kang et al.2 | 2012 | Male | 51 | No | Dyspnea, chest pain, and fever | Septic AVT with septic PE | Antibiotics | No |
| Smith et al.17 | 2011 | Male | 33 | Congenital inferior vena cava abnormality with azygos continuation | Nephritic colic and hematuria | AVT | Indefinite anticoagulation | No |
| Yang et al.5 | 2011 | Female | 75 | AVA | Asthenia and chronic cough | ATV and PE | Heparin (4 days) followed by warfarin for 2 months | No |
| Ishikura et al.6 | 2010 | Female | 51 | AVA | Asymptomatic | AVT | Surgical resection | No |
| Probst et al.19 | 2010 | Female | 81 | Renal carcinoma | Not specified | AVT, thrombosis of the IVC and the left renal vein | Not specified | Not specified |
| Gnanamuthu et al.7 | 2008 | Male | 73 | AVA | Cough and mild dysphagia | AVT | Surgical resection | No |
| Irurzun et al.8 | 2008 | Male | 77 | Endovascular treatment of AVA | Cough, wheezing, and hiccups | AVT | None | No |
| Nakamura et al.9 | 2007 | Female | 37 | AVA | Palpitations and chest pain | ATV and PE | Ineffective anticoagulation and urgent surgical resection | No |
| Gomez et al.10 | 2004 | Male | 20 | AVA | Chest pain | AVT | Surgical resection | No |
| Icard et al.11 | 1999 | Male | 68 | AVA | Chest pain | AVT | Surgical resection | No |
AVA: azygos vein aneurysm; AVT: azygos vein thrombosis; DVT: deep vein thrombosis; ERCP: endoscopic retrograde cholangiopancreatography; IDA: inhaled drug abuser; IVC: inferior vena cava; PICC: peripherally inserted central catheter; PE: pulmonary embolism; SVC: superior vena cava; VCS: vena cava syndrome.
Isolated AVT was conventionally considered as asymptomatic deep vein thrombosis (DVT) in an unusual site, until it was associated with complications such as PE or VCS. In our patient, the syncopal episode, together with the clinical picture suggestive of VCS, confirmed the presence of AVT and HVT in the imaging tests. In most cases included in the review, AVT was symptomatic (84.2%), with chest pain being the most frequent symptom (42.1%), followed by cough (26%) and dyspnea (21%). The most frequent complications included thrombosis in other sites (15.7%),15,16,19 followed by PE (10.5%),5,9 septic PE (10.5%),2,13 VCS (5.2%),16 and transient antiphospholipid syndrome (5.2%).12
Chest CT-angiogram is the test of choice for the diagnosis of AVT, while a CT-venography should be considered if VCS is suspected. In thrombosed AVAs, the use of dynamic multidetector CT with ECG to assess myocardial damage has been proposed.5
As AVT is considered thrombosis in an unusual site, the treatment recommendations have been extrapolated from published studies on VTE.20 Almost one third (31.6%) of AVT cases were treated with anticoagulants, while in 1 case, anticoagulation was ineffective and surgical resection was required.9 Antibiotics are the first line of treatment in AVT associated with infection and/or septic PE,2,12,13 although anticoagulation in these cases may be controversial. Some cases of endovascular treatment, thrombectomy, and removal of infected devices have been reported.11,12
Despite the association of AVA with AVT and PE, treatment of asymptomatic aneurysm is not well established, and experts recommend radiological follow-up.4 Furthermore, there is no established consensus for asymptomatic thrombosed AVA, and treatment can range from indefinite anticoagulation to surgical resection to prevent PE. In our series, 36.8% of the symptomatic and/or thrombosed AVAs underwent surgical resection without subsequent anticoagulation.1,4,6,7,9–11 In VCS secondary to AVT, the first-line treatment is anticoagulation. It is effective in up to 88% of patients and can be combined with percutaneous stent placement for immediate symptomatic relief and chemotherapy and/or radiotherapy with long-term curative or palliative intent. According to experts, anticoagulation for the treatment of VCS due to thrombosis should be maintained indefinitely.21 In the series reviewed, there were no deaths in follow-up.
In conclusion, the importance of this clinical case lies in the rare presentation of a DVT in an unusual site in an anatomically normal azygos system. This is an extremely rare presentation in isolation that we were able to diagnose and treat promptly due to the clinical presentation suggestive of VCS.
Conflict of interestsLuis Jara Palomares has received honoraria for speaking engagements and for travel and accommodation from Rovi, Pfizer, Menarini, Leo-Pharma and GSK, unrelated to this manuscript. Maria Isabel Asensio Cruz has received honoraria for travel and accommodation from Rovi, Novartis and Teva, unrelated to this manuscript. Raquel Morillo Guerrero has received honoraria for speaking engagements and for travel and accommodation from Pfizer, Menarini and GSK, unrelated to this manuscript. The other authors state that they have no conflict of interests.
Please cite this article as: Lopez-Bauza A, Jara-Palomares L. Trombosis sintomática de la vena ácigos: caso clínico y revisión de la literatura. Arch Bronconeumol. 2021;57:506–509.