Silicones are a group of polydimethylsiloxane polymers with differing viscosity, depending on their chain length. They are widely used in cosmetic and reconstructive surgery due to their supposed physical stability and lack of immunogenicity. However, these compounds are not inert, and numerous local and systemic complications associated with their use have been reported.1–3
Most cases of pulmonary toxicity described in the literature are associated with subcutaneous injections of liquid silicone, and this practice is currently banned by the FDA. In contrast, systemic complications due to silicone gel prostheses are exceptionally rare.1 We report the case of subacute pneumonitis caused by silicone in a patient with breast implants.
A 55-year-old woman, non-smoker, with a history of primary biliary cirrhosis, who had received bilateral breast implants 10 years previously. She presented in the pulmonology clinic with a 3-month history of symptoms including irritative cough, low-grade fever, pleuritic chest pain, dyspnea on moderate exertion, asthenia, and loss of appetite. Of note on physical examination were tachypnea, 24breaths/min and crackles in upper left fields on auscultation. Arterial blood gases, complete blood count and serum biochemistry results were normal.
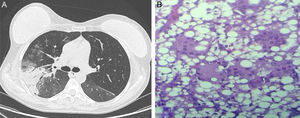
On chest radiograph, ground glass opacities and airspace consolidation in both lung bases and periphery were observed. The initial diagnosis was pneumonia, and the patient began antibiotic treatment with moxifloxacin. However, her progress was slow, so she was admitted for further tests. A fiberoptic bronchoscopy was performed, which revealed no pathological findings. Chest computed tomography revealed new areas of parenchymal consolidation in the upper right lobe (Fig. 1A). Finally, the patient underwent a surgical lung biopsy by videoassisted thoracoscopy. The pathology study gave a diagnosis of foreign body giant cell reaction, with macrophages containing lipid vacuoles (Fig. 1B). Magnetic resonance imaging of the breast confirmed the intra- and extracapsular rupture of the right breast prosthesis. The prosthesis was removed surgically and oral corticosteroid treatment was initiated, after which the patient's progress was favorable.
Silicone implants are increasingly used in breast surgery both for reconstructive and cosmetic reasons. Migration of silicone after transplant generally occurs after rupture of the prosthesis, although silicone can also seep through an intact shell.4
The first case of silicone pneumonitis was described in 1975, and since then similar case series have been reported, mostly due to subcutaneous injections of liquid silicone. The pathogenesis of this disease is unknown, but the most accepted hypotheses suggest hematogenous or lymphatic dissemination of the silicone. Two clinical courses have been described: the acute form, which occurs with sudden-onset dyspnea, fever and chest pain; and the latent form, with onset 6 months after the application of the biopolymer, that occurs with more simmering symptoms.5
The definitive diagnosis can be achieved with transbronchial or open biopsy, although the presence of macrophages with intracytoplasmic lipid inclusions in the bronchoalveolar lavage is characteristic of silicone pneumonitis, and may obviate the need for a biopsy when clinical suspicion is well-founded. Acute phase treatment entails respiratory support, with administration of high-flow oxygen and mechanical ventilation in more severe cases.
In conclusion, silicone pneumonitis is a rare and potentially severe complication that may occur after the application of silicone for cosmetic purposes. This complication occurring in association with a gel prosthesis is even rarer, although it is important to include it in the differential diagnosis of patients with breast implants who present inflammatory pulmonary processes.
Please cite this article as: Hernández MJG, Milena GL, Carazo ER. Neumonitis subaguda por silicona tras la rotura silente de un implante mamario. Arch Bronconeumol. 2016;52:397–398.