In recent years an increase in the prevalence of colonization and infection by Scedosporium spp. in patients with cystic fibrosis (CF) has been observed. In this article, we study the frequency of isolation of Scedosporium spp. in an adult CF Unit, analyzing characteristics of the patients and predisposing factors.
MethodsA retrospective observational study was conducted in 87 adult CF patients in whom the presence of positive culture for Scedosporium spp. was tested for a 5-year period (January 2012–July 2017). We recorded the following clinical variables: age, sex, body mass index, genotype, presence of pancreatic insufficiency, bacterial colonization, lung function, other complications, exacerbations and treatment, and the modified Bhalla score from the last high-resolution computed tomography. Results were analyzed with IBM SPSS Statistics Version 22.0 software.
ResultsScedosporium spp. was isolated in 25.3% of patients. In the bivariate analysis, these patients showed a higher rate of Pseudomonas aeruginosa infection, worse score in the Bhalla classification (highlighting the following items: bronchiectasis, mucus plugs and bronchial generations), a slight decrease in the lung diffusion capacity and more frequently received inhaled antibiotics. In the logistic regression multivariate analysis, only the bronchial generations item was significant.
ConclusionScedosporium spp. must be considered an emerging opportunistic pathogen in patients with CF whose clinical involvement, risk factors or need for treatment is unknown.
En los últimos años se observa un aumento de la prevalencia de colonización e infección por Scedosporium spp. en pacientes con fibrosis quística (FQ). En el presente estudio se registra la frecuencia de aislamiento de Scedosporium spp. en una Unidad de FQ de adultos, analizándose las características de los pacientes y los factores predisponentes.
MétodosSe realizó un estudio observacional retrospectivo en 87 pacientes adultos con FQ en los que se valoró la presencia de cultivo positivo para Scedosporium spp. durante 5 años (enero de 2012-julio de 2017). Se recogieron las siguientes variables clínicas: edad, sexo, índice de masa corporal, genotipo, presencia de insuficiencia pancreática, colonizaciones bacterianas, función pulmonar, complicaciones, exacerbaciones y tratamiento, así como puntuación Bhalla modificada de la última tomografía computarizada axial de alta resolución. Los resultados se analizaron con el paquete estadístico IBM SPSS Statistics Version 22.0.
ResultadosEn un 25,3% de los pacientes se aisló Scedosporium spp. En el análisis bivariante se observó en estos enfermos más frecuencia de Pseudomonas aeruginosa, peor puntuación en la clasificación de Bhalla (destacando los ítems presencia de bronquiectasias, tapones mucosos y generaciones bronquiales), un descenso leve en la capacidad de difusión pulmonar (DLCO) y que recibían con más frecuencia antibioterapia inhalada. En el análisis multivariante de regresión logística únicamente el ítem generaciones bronquiales fue significativo.
ConclusionesScedosporium spp. debe considerarse un patógeno oportunista emergente en pacientes con FQ del que se desconoce su implicación clínica, factores de riesgo o necesidad de tratamiento.
The study of respiratory infections in patients with cystic fibrosis (CF) is complex due to the multitude of microorganisms involved. In addition to chronic bacterial bronchial infection, patients with CF are predisposed to fungal colonization due to the ability of some fungi to multiply in the lower respiratory tract and the frequent antibiotic cycles they receive for the control of their disease.1 While the clinical relevance of bacteria such as Pseudomonas aeruginosa (P. aeruginosa) and Staphylococcus aureus (S. aureus) in pulmonary decline is clearly established, the role of some fungi is yet to be determined.2
Despite the fact that Scedosporium spp. is a very rare human pathogen, it is the second most common filamentous fungus in CF patients after Aspergillus spp. Unlike Aspergillus spp., its spores are rarely found in the environment and the mechanisms of transmission and chronic colonization are unclear.3 The inhalation of spores has little effect in healthy subjects whose immune system is functioning properly. In contrast, in patients with chronic respiratory diseases, such as bronchiectasis, the fungi are more persistent, due to impaired mucociliary clearance, thick secretions, and their ability to evade the host's immune system.
Although a widely ranging prevalence of colonization by Scedosporium spp. has been described in patients with CF, one reason for the increased detection rate in recent years is improved screening procedures. Few studies have been published on the real prevalence of co-infection by fungi and bacteria in CF. In most cases, fungi are isolated together with bacteria, such as Haemophilus influenzae (H. influenzae) or P. aeruginosa, which makes it difficult to determine its real pathogenic impact, although they are known to be associated with a persistent inflammatory airway response.2 To date, few studies have analyzed the clinical impact and possible predisposing factors for colonization by Scedosporium spp. in patients with CF.4
Due to recent changes in taxonomy, Scedosporium apiospermum complex is now considered a complex formed by 5 different species: Scedosporium apiospermum (S. apiospermum), Scedosporium boydii, Scedosporium aurantiacum, Scedosporium minutispora and Scedosporium dehoogii. Scedosporium prolificans, however, is genetically different from other species of Scedosporium spp., as recently demonstrated by Lackner et al.5 For this reason, it is considered a distinct genus and has been reassigned to the genus Lomentospora, and is now called Lomentospora prolificans.
The objective of this study was to analyze the frequency of isolates of Scedosporium spp. and their predisposing factors in a cohort of patients with a diagnosis of CF monitored in an adult unit.
Materials and MethodsWe collected all Scedosporium spp. isolated from respiratory secretions of patients treated in the Adult CF Unit of the Hospital Universitario de La Princesa, Madrid (Spain) over the 5-year period between January 2012 and July 2017. This department provides healthcare to a total of 87 patients, and detailed charts are available for each case. Check-ups were monthly or quarterly, depending on severity, and patients also attended in case of clinical exacerbation. Their general status, lung function, antibiotic therapeutic control, and microbiological findings obtained in respiratory samples were evaluated at each visit. Patients were classified according to whether Scedosporium spp. had been previously isolated or not.
Sputum samples were collected at each patient visit. Given the viscosity of the samples, the usual microbiological processing involved prior homogenization with acetylcysteine. Quantitative inoculation was performed to obtain a count of the different pathogens and facilitate the recognition of the different bacterial morphotypes. Selective media used were Mannitol Salt, MacConkey, and Burkholderia cepacia Selective Agar. In addition, chocolate agar with bacitracin, Saboureaud agar, and blood agar were inoculated as a general medium for the total count of the respiratory microflora. The incubation period was 5 days. Filamentous fungi isolated were identified by macroscopic/microscopic structural analysis and confirmed with MALDI-TOF (Bruker-Daltonics).
We analyzed the first isolate of Scedosporium spp. from each patient, and presence or absence in successive cultures. Two groups were defined according to the microbiological results: patients who had at least one positive culture for Scedosporium spp. in respiratory secretions during the entire study period, and those in whom Scedosporium spp. was never isolated. The definition of bronchial fungal infection is not clearly established. However, although there is no consensus definition, a bronchial infection was defined as isolation from at least 3 separate cultures at intervals of at least 1 month over a period of 6 months.
To assess pulmonary function, we studied forced expiratory volume in 1 second (FEV1) and FEV1%, coinciding with the first isolation of Scedosporium spp. We also evaluated respiratory exacerbations (RE), defined as increased baseline symptoms requiring additional antibiotics; REs were considered mild-moderate when oral (vo) antibiotic treatment was required, and severe if intravenous (iv) antibiotics were needed. Data on cycles of oral or intravenous antibiotic therapy in the year before (retrospectively) and after the first isolation of Scedosporium spp. were collected.
The following clinical variables were collected simultaneously: gender of participants, age, body mass index (BMI); genotype, according to information from the genetic study, classified into three groups: homozygous F508del, heterozygous F508del, and other mutations; existence of pancreatic insufficiency, defined as need for pancreatic enzymes with lower levels of fecal elastase (<200μg/g); CF-related diabetes (CFRD) with fasting hyperglycemia: fasting blood glucose levels greater than 126mg/dl and above 200mg/dl at 2h; allergic bronchopulmonary aspergillosis (ABPA); and chronic bacterial infection, defined according to the Leeds criteria: isolation of bacteria in more than 50% of samples cultured in the previous 12 months, classifying patients as single isolation or chronic infection.6 The modified Bhalla score was calculated from the high resolution axial computed tomography (HRCT) performed closest to the last isolation. This system is used to assess the degree of pulmonary involvement and the course of the lung damage caused by the disease, depending on various radiological findings. The overall score is obtained by subtracting points from a maximum score of 25, which would be the best possible radiological situation.7 The presence of severe hemoptysis was recorded, defined as greater than 400ml/day or 150ml/h, regardless of whether it was treated conservatively or if bronchoscopy or embolization was required.
Chronic treatment was also evaluated, defined as treatment administered to the patient for a period ≥3 months, including: macrolides, aerosol antibiotics (colistin, tobramycin, aztreonam, or other), oral corticosteroids, inhaled corticosteroids, ibuprofen, oral antibiotic, aerosol with DNase, 0.9% hypertonic and physiological saline solution.
The results were analyzed using the IBM SPSS Statistics package version 22.0. Quantitative variables were analyzed descriptively by calculating means and standard deviations, and qualitative variables were described by frequencies and percentages. Student's t and Mann–Whitney-U tests were used to compare continuous variables, according to normality. The Chi-squared test was used to compare categorical variables. The effect of the dependent variable was evaluated using the odds ratio (OR) and its confidence interval, adjusting for the different covariates with a conditional logistic regression model. A p-value of ≤0.05 was considered statistically significant.
ResultsDuring the 5-year study period, at least 1 positive culture for Scedosporium spp. was obtained from 22 of the 87 patients seen in the CF unit. Lomentospora prolificans was isolated in 5 patients, Scedosporium apiospermum complex in 12, and both were isolated in 5 patients. With regard to isolates per patient, the mean number of positive cultures in each patient was 10.7±16.3. Scedosporium spp. was isolated only once in 4 patients, while in the remaining 18 it was isolated on more than 2 occasions. Specifically, 7 patients presented chronic bronchial infection due to Scedosporium spp., which was subsequently isolated during a period of between 6 months and the present.
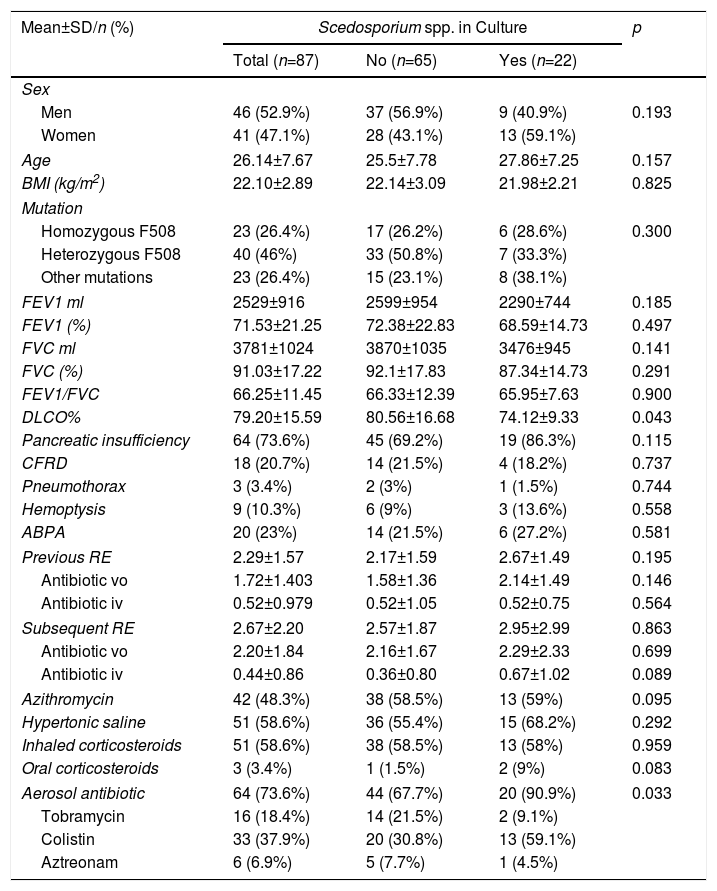
With regard to the clinical characteristics of patients included in the study, the average age was 26.14 years, ranging between 18.5 and 34 years of age (Table 1). The genotype of the most prevalent mutation was heterozygous F508. There were no statistically significant differences according to sex or lung function in the 2 groups of patients with and without Scedosporium spp., although patients with positive culture for Scedosporium spp. showed a slight decrease in lung diffusing capacity (DLCO) (p=0.043).
Patient Clinical Characteristics.
| Mean±SD/n (%) | Scedosporium spp. in Culture | p | ||
|---|---|---|---|---|
| Total (n=87) | No (n=65) | Yes (n=22) | ||
| Sex | ||||
| Men | 46 (52.9%) | 37 (56.9%) | 9 (40.9%) | 0.193 |
| Women | 41 (47.1%) | 28 (43.1%) | 13 (59.1%) | |
| Age | 26.14±7.67 | 25.5±7.78 | 27.86±7.25 | 0.157 |
| BMI (kg/m2) | 22.10±2.89 | 22.14±3.09 | 21.98±2.21 | 0.825 |
| Mutation | ||||
| Homozygous F508 | 23 (26.4%) | 17 (26.2%) | 6 (28.6%) | 0.300 |
| Heterozygous F508 | 40 (46%) | 33 (50.8%) | 7 (33.3%) | |
| Other mutations | 23 (26.4%) | 15 (23.1%) | 8 (38.1%) | |
| FEV1 ml | 2529±916 | 2599±954 | 2290±744 | 0.185 |
| FEV1 (%) | 71.53±21.25 | 72.38±22.83 | 68.59±14.73 | 0.497 |
| FVC ml | 3781±1024 | 3870±1035 | 3476±945 | 0.141 |
| FVC (%) | 91.03±17.22 | 92.1±17.83 | 87.34±14.73 | 0.291 |
| FEV1/FVC | 66.25±11.45 | 66.33±12.39 | 65.95±7.63 | 0.900 |
| DLCO% | 79.20±15.59 | 80.56±16.68 | 74.12±9.33 | 0.043 |
| Pancreatic insufficiency | 64 (73.6%) | 45 (69.2%) | 19 (86.3%) | 0.115 |
| CFRD | 18 (20.7%) | 14 (21.5%) | 4 (18.2%) | 0.737 |
| Pneumothorax | 3 (3.4%) | 2 (3%) | 1 (1.5%) | 0.744 |
| Hemoptysis | 9 (10.3%) | 6 (9%) | 3 (13.6%) | 0.558 |
| ABPA | 20 (23%) | 14 (21.5%) | 6 (27.2%) | 0.581 |
| Previous RE | 2.29±1.57 | 2.17±1.59 | 2.67±1.49 | 0.195 |
| Antibiotic vo | 1.72±1.403 | 1.58±1.36 | 2.14±1.49 | 0.146 |
| Antibiotic iv | 0.52±0.979 | 0.52±1.05 | 0.52±0.75 | 0.564 |
| Subsequent RE | 2.67±2.20 | 2.57±1.87 | 2.95±2.99 | 0.863 |
| Antibiotic vo | 2.20±1.84 | 2.16±1.67 | 2.29±2.33 | 0.699 |
| Antibiotic iv | 0.44±0.86 | 0.36±0.80 | 0.67±1.02 | 0.089 |
| Azithromycin | 42 (48.3%) | 38 (58.5%) | 13 (59%) | 0.095 |
| Hypertonic saline | 51 (58.6%) | 36 (55.4%) | 15 (68.2%) | 0.292 |
| Inhaled corticosteroids | 51 (58.6%) | 38 (58.5%) | 13 (58%) | 0.959 |
| Oral corticosteroids | 3 (3.4%) | 1 (1.5%) | 2 (9%) | 0.083 |
| Aerosol antibiotic | 64 (73.6%) | 44 (67.7%) | 20 (90.9%) | 0.033 |
| Tobramycin | 16 (18.4%) | 14 (21.5%) | 2 (9.1%) | |
| Colistin | 33 (37.9%) | 20 (30.8%) | 13 (59.1%) | |
| Aztreonam | 6 (6.9%) | 5 (7.7%) | 1 (4.5%) | |
ABPA: Allergic bronchopulmonary aspergillosis; BMI, body mass index; CFRD: CF-related diabetes; DLCO: diffusing capacity of the lung; FEV1: forced expiratory volume in 1 second; FVC: forced vital capacity; iv: intravenous; n: number; RE: respiratory exacerbations; SD: standard deviation; vo: oral.
Most patients in our series were receiving chronic treatment (93.1%), and statistically significant differences were observed only in the use of inhaled antibiotics (p=0.033). The inhaled antibiotic most frequently prescribed in patients with Scedosporium spp. was inhaled colistin (Table 1).
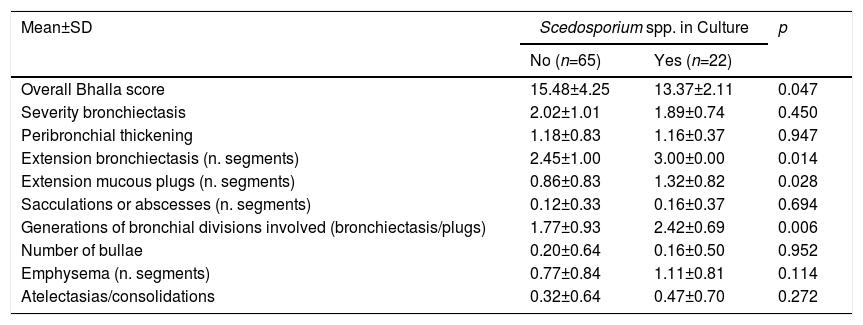
With regard to prognostic factors, the overall Bhalla score was worse in patients in whom Scedosporium spp. was isolated, with statistically significant results (p=0.047). In the bivariate analysis, the Bhalla items were significant for: extension of bronchiectasis, extension of bronchial mucous plugs and generations affected by bronchiectasis (Table 2).
Modified Bhalla Score.
| Mean±SD | Scedosporium spp. in Culture | p | |
|---|---|---|---|
| No (n=65) | Yes (n=22) | ||
| Overall Bhalla score | 15.48±4.25 | 13.37±2.11 | 0.047 |
| Severity bronchiectasis | 2.02±1.01 | 1.89±0.74 | 0.450 |
| Peribronchial thickening | 1.18±0.83 | 1.16±0.37 | 0.947 |
| Extension bronchiectasis (n. segments) | 2.45±1.00 | 3.00±0.00 | 0.014 |
| Extension mucous plugs (n. segments) | 0.86±0.83 | 1.32±0.82 | 0.028 |
| Sacculations or abscesses (n. segments) | 0.12±0.33 | 0.16±0.37 | 0.694 |
| Generations of bronchial divisions involved (bronchiectasis/plugs) | 1.77±0.93 | 2.42±0.69 | 0.006 |
| Number of bullae | 0.20±0.64 | 0.16±0.50 | 0.952 |
| Emphysema (n. segments) | 0.77±0.84 | 1.11±0.81 | 0.114 |
| Atelectasias/consolidations | 0.32±0.64 | 0.47±0.70 | 0.272 |
SD: standard deviation; n: number.
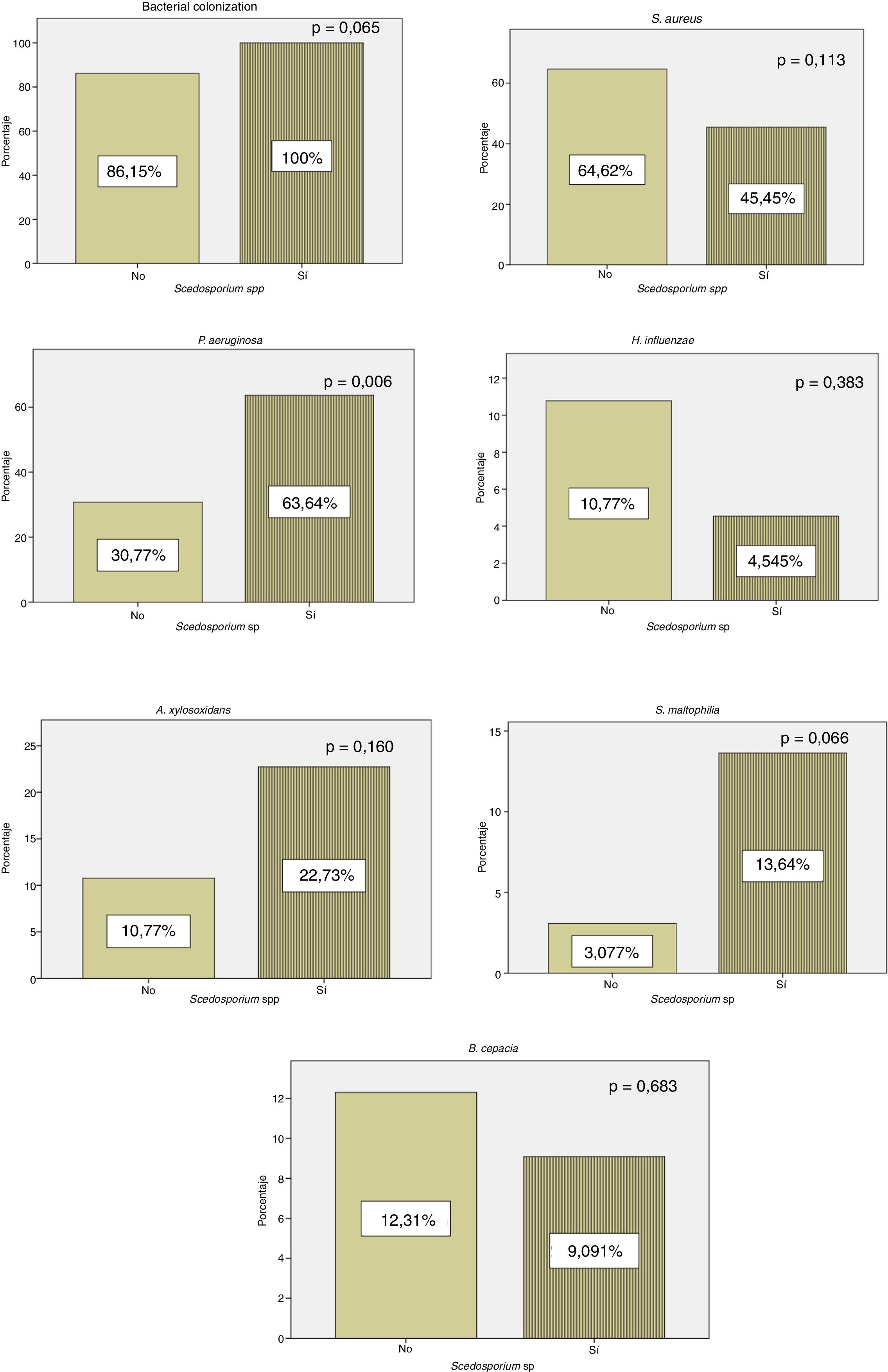
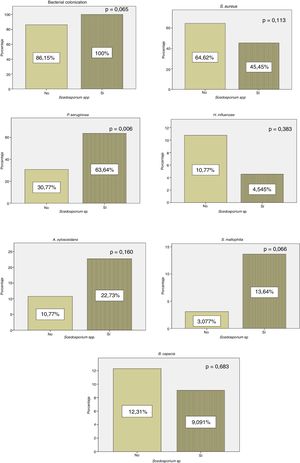
All patients with positive culture for Scedosporium spp. presented concomitant colonization by other microorganisms, while 56 (86.15%) patients in whom Scedosporium spp. was not isolated were co-infected by another pathogen. Our results showed a significantly higher rate of chronic P. aeruginosa colonization in patients with a positive culture compared to those without (p=0.006) (Fig. 1).
With regard to treatment, only 3 of 22 patients were treated for Scedosporium spp., with an average treatment duration of 16.5 months, ranging between 3 and 30 months. The prescribed treatment in the 3 cases was voriconazole, voriconazole+terbinafine, or itraconazole+terbinafine.
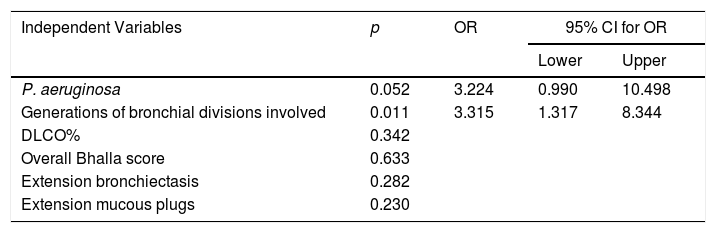
Risk factors that were statistically significant (p≤0.05) after the bivariate analysis were included in the multivariate analysis, as follows: P. aeruginosa, DLCO%, overall Bhalla score, bronchial generations affected by bronchiectasis, extension of bronchiectasis, and extension of mucous plugs. After the multivariate analysis, only bronchial generations affected by bronchiectasis showed statistical significance (OR: 3.315; 95% CI: 1.317–8.344, p=0.011) (Table 3).
Logistic Regression Analysis.
| Independent Variables | p | OR | 95% CI for OR | |
|---|---|---|---|---|
| Lower | Upper | |||
| P. aeruginosa | 0.052 | 3.224 | 0.990 | 10.498 |
| Generations of bronchial divisions involved | 0.011 | 3.315 | 1.317 | 8.344 |
| DLCO% | 0.342 | |||
| Overall Bhalla score | 0.633 | |||
| Extension bronchiectasis | 0.282 | |||
| Extension mucous plugs | 0.230 | |||
CI: confidence interval; OR: odds ratio.
This is one of the few studies to assess the prevalence of Scedosporium spp. in a series of CF patients and analyze the possible risk factors. It highlights the high prevalence of this fungus, and its association with P. aeruginosa and greater structural and functional gas exchange damage. We cannot conclude whether these findings are a result of the fungus or if they are a consequence of the disease course, which makes this fungus appear more frequently in this type of patient. Notwithstanding, we observed that the prevalence of Scedosporium spp. may increase in patients with involvement of a greater number of bronchial generations.
CF patients have a high predisposition for colonization-chronic bronchopulmonary infection, the main cause of high morbidity and early mortality of these patients.3 While the clinical relevance of bacteria, such as P. aeruginosa and S. aureus, in pulmonary impairment is clearly established, the role of some filamentous fungi (with the exception of A. fumigatus) is yet to be determined.2 In our study, patients in whom Scedosporium spp. was isolated had a worse Bhalla score, especially with regard to mucous plugs, which might explain why DLCO decline was greater than FEV1 decline.
Recently, Schwarz et al.8 conducted a review of the epidemiology of fungi in CF patients in different European countries. They detected significant geographical differences and emphasized the need for more local studies. In the Netherlands, Engel et al.9 reported that Aspergillus spp. is the most common filamentous fungus. These data are consistent with ours, which showed a rate of Aspergillus spp. isolation of 45.9% (data not shown). The overall prevalence of Scedosporium spp. has been estimated at over 14%, but prevalence in our study was 25.3%, making it the second most common filamentous fungi in the airways of CF patients, after A. fumigatus.10 In German cohorts, the estimated prevalence was between 3.1% is11 and 5.3%.12 In France, it was 8.6%,13 and in Australia it ranged from 17.4% to 25%.14,15 This fungus is generally ubiquitous in contaminated water and soil, which is why young patients have an increased risk of colonization as they tend to spend more time outdoors, making it easier for them to become infected. While the mechanisms of transmission and colonization are unclear, a greater geographic and climatic predisposition is emerging.4Lomentospora prolificans, specifically predominates in Australia, the United States, and Spain.
Another factor that may influence their heterogeneous prevalence is the lack of standardized testing procedures and the fact that other filamentous fungi like A. fumigatus grow more rapidly than Scedosporium spp. in non-selective media. The recent introduction of semiselective culture media that inhibit the rapid growth of A. fumigatus has allowed colonies that grow more slowly to be detected. Molecular techniques such as loop-mediated isothermal amplification (LAMP) reverse hybridization can be powerful alternatives to culture media, increasing the rate of detection in sputum samples.16 Both fungi can be grown in this manner, and Aspergillus spp. was also isolated in 9 of the 22 patients with Scedosporium spp.
In our series, P. aeruginosa colonization is more frequent in patients with positive culture for Scedosporium spp. (Fig. 1). These results are consistent with the data of Schwarz et al.,4 who considered P. aeruginosa colonization as a probable risk factor for Scedosporium spp. isolation. According to Schwarz et al.,4H. influenzae rates were lower in our cohort in patients with a positive culture for Scedosporium spp. (Fig. 1). In this series, we found a statistically significant relationship between exacerbations and H. influenzae colonization. This pathogen has the capacity to produce a biofilm that helps it persist in the respiratory system. It is suggested that the lower rates of H. influenzae in patients with CF and positive culture for Scedosporium spp. are associated with repeated antibiotic treatment.
Treating Scedosporium spp. is a challenge because in vitro studies show high resistance or low sensitivity to most available antifungal treatments. The first cases of colonization by Scedosporium spp. were detected after A. fumigatus was eradicated with itraconazole.17 At the start of treatment, it is vital to recognize the species of Scedosporium spp., as different strains have shown different susceptibilities in vitro. Therefore, the logical approach is to start treatment with 2 or even 3 anti-fungal agents, to achieve better therapeutic response and also to prevent the development of resistance. Voriconazole has shown the best sensitivity in vitro in the treatment of Scedosporium spp., and this is the drug we used to treat most of our patients. However, recent treatment guidelines from the Royal Brompton Hospital (2017)18 discourage the use of voriconazole due to its side effects, high photosensitivity despite the use of sunscreen, and risk of liver toxicity. For this reason, the better tolerated posaconazole is currently recommended in Scedosporium apiospermum complex infection. As Lomentospora prolificans is highly resistant to all anti-fungal agents, the combination of posaconazole and terbinafine is recommended. With regard to aerosol therapy, Sole et al.19 have recently reported 3 cases of transplanted patients with invasive disease due to S. apiospermum who received nebulized posaconazole in compassionate use. In their experience, nebulized posaconazole was effective and showed a similar tolerance to other nebulized antifungals. The authors also consider it an alternative therapeutic option if progress with conventional treatment is inadequate, or if the infection is caused by highly resistant fungi. Treatment duration after starting has not been established.
The question of whether colonization of the respiratory tract by Lomentospora prolificans in CF patients is a contraindication for lung transplantation is still under discussion.20 It has been shown that colonized patients have a worse prognosis after transplantation.21 It has also been reported to cause invasive disease in immunocompromised lung transplant recipients. The time for determining invasive fungal infection is 12 months after transplantation. A 3.5% rate of Scedosporium spp. infection after lung transplantation has been described in 1 study.22
The main limitations of our study were that all patients were recruited from a single center and the number of patients included was low, so multicenter studies would be required to determine the real prevalence of this fungus, and to clarify some issues.
We conclude that Scedosporium spp. is an emergent fungus in CF patients, raising many questions regarding its clinical implications, risk factors, and need for treatment, which will have to be answered by more studies. For now, we recommend that these patients are closely monitored.
Conflict of InterestsThe authors state that they have no conflict of interests.
Please cite this article as: Erro Iribarren M, Girón Moreno RM, Diab Cáceres L, Pastor Sanz MT, Buendía Moreno B, Alarcón Cavero T, et al. Estudio de una cohorte de paciente con fibrosis quística y Scedosporium spp. Arch Bronconeumol. 2019;55:559–564.